Recce Pharmaceuticals Ltd is pleased to provide an update on the utilization of RECCE® 327 as a gel formulation, or RECCE® 327 Gel, by a qualified medical practitioner for patients with antibiotic-resistant Gram-positive and Gram-negative bacterial infections under the Therapeutic Goods Administration Special Access Scheme Category A, a compassionate-use provision.
- RECCE® 327 as a gel formulation (R327G) demonstrated a positive clinical response in the treatment of multiple antibiotic-resistant infections under Therapeutic Goods Administration (TGA) Special Access Scheme (SAS) Category A
- In an ex-vivo burn wound study, R327G showed a 4 to 5-log reduction (>99.99%) against Methicillin-resistant bacteria, Staphylococcus aureus (S. aureus)
- Clinical trial preparations are underway across multiple unmet medical needs
SYDNEY, Australia, Aug. 10, 2023 (GLOBE NEWSWIRE) -- Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q), the Company developing a new class of synthetic anti-infectives, is pleased to provide an update on the utilization of RECCE® 327 as a gel formulation, or RECCE® 327 Gel (R327G), by a qualified medical practitioner for patients with antibiotic-resistant Gram-positive and Gram-negative bacterial infections under the Therapeutic Goods Administration (TGA) Special Access Scheme (SAS) Category A, a compassionate-use provision.
“Antibiotic resistance is globally recognized as one of the greatest threats to human health today,” said James Graham, Chief Executive Officer of Recce Pharmaceuticals. “To see Recce making a difference for patients in such great medical need is a significant sign of new hope in the fight against drug-resistant superbugs. We look forward to building upon these successes in present and future clinical trials.”
Patients have been treated under the SAS-Category A, a notification pathway that health practitioners can access on behalf of patients who are seriously ill with a condition from which death is likely to occur within a matter of months or from which premature death is expected to occur in the absence of early treatment and does not constitute a clinical trial.1
R327 and R327G are experimental compounds, not market approved for use in humans, with safety and efficacy to be determined by current clinical studies. The results shown below must be considered anecdotal; however, they are presented in the interest of continuous disclosure obligations and are not part of any current clinical trials.
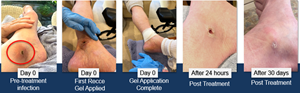
Patient Case Study Example A
A 70–75-year-old male who received a puncture wound from a metal spike injury was unresponsive to all prior antibiotics, and with the infection spreading, he was preparing for surgical intervention.
After 24 hours and only one dosing application of R327G, the infection had clinically responded, with the patient not requiring a wound debridement prior to treatment. Furthermore, the redness and swelling of the wound were reduced, with no reported stinging at any point. At 30 days post-treatment, the wound had successfully healed and closed.
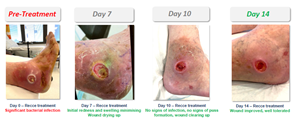
Patient Case Study Example B
A 72-year-old male with type 2 diabetes, peripheral vascular disease, and peripheral neuropathy was unresponsive to all prior antibiotics.
Prior to treatment with R327G, the patient showed significant bacterial infection, redness, and swelling. Upon applying R327G, the initial redness and swelling had minimized after seven days, with the wound healing and drying up. On day 10, the patient showed no signs of infection and pus formation, and the wound continued to heal. On day 14, the wound significantly improved, demonstrating R327G to be well-tolerated. Surgical intervention was averted, such as limb amputation, which is common in patients with diabetes.
Other patient case studies have not been disclosed for matters of confidentiality; however, they include the successful treatment of but are not limited to necrotizing fasciitis (flesh-eating disease), osteomyelitis (bone infection), and complex skin structure bacterial infections.
Preclinical results support the potential of R327 as a gel formulation
An independent clinical research organization conducted an animal study where multiple R327G formulations were tested for efficacy against Methicillin-resistant S. aureus (MRSA), which is listed as “High” on the World Health Organization’s Priority Pathogen list of antibiotic-resistant bacteria, using an ex-vivo porcine (pig) skin model. R327 as an intravenous (IV) formulation, or R327 IV, was also included in this study and is currently being evaluated in human clinical trials.
After 24 hours, R327G achieved a 4 to 5-log reduction (99.99% - 99.999% reduction) in all formulations and had the greatest overall efficacy against MRSA. MRSA infections are one of the leading causes of hospital-acquired infections and are commonly associated with significant morbidity, mortality, length of stay, and cost burden.2 MRSA most often causes skin infections and, if left untreated, can become severe and cause sepsis.3
| Treatment | Log10 Reduction from Control (Mean ± SD) | |
1hr |
Control | N/A |
| RECCE® 327 I.V. | 3.04 ± 1.20 | |
| RECCE® 327 Gel (1.0% CMC) | 2.10 ± 1.23 | |
| RECCE® 327 Gel (2.0% CMC) | 0.90 ± 0.13 | |
24hr |
Control | N/A |
| RECCE® 327 I.V. | 4.97 ± 0.11 | |
| RECCE® 327 Gel (1.0% CMC) | 4.32 ± 0.81 | |
| RECCE® 327 Gel (2.0% CMC) | 5.07 ± 0.26 | |
Numerical values for Methicillin-resistant Staphylococcus aureus Log10 Reduction
The Company engaged Linnaeus Bioscience, based in San Diego, CA, to test R327G against antimicrobial activity in different formulations.
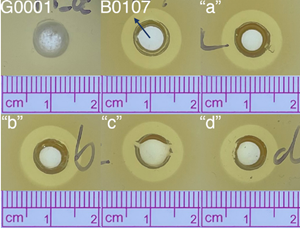
The image below shows a clearing in a lawn of Escherichia coli (E. coli) (ATCC 25922) on solid LB-agar media. Each of the gel formulations (R327G “a, b, c and d”) produced a zone of clearing approximately 13mm in diameter, indicating each formulation is active and demonstrates similar potency to R327 IV (B0107) on solid media.
Monitored dispersion is observed outside the dark ring. G0001 (placebo control) had no effect on the E. coli; however, R327 IV and R327G successfully dispersed outwards through the bacterial lawn.
The above topical results may lead to the progression of R327G to Stage 2 investigations with the burn wound team at Fiona Stanley Hospital, including the potential use among patient populations.
Separately, clinical trial applications for the topical utilization of R327G in common and complicated skin and soft tissue infections are progressing in line with these developments.
About Recce Pharmaceuticals Ltd
Recce Pharmaceuticals Ltd (ASX: RCE, FSE: R9Q) is developing a New Class of Synthetic Anti-Infectives designed to address the urgent global health problems of antibiotic-resistant superbugs and emerging viral pathogens.
Recce’s anti-infective pipeline includes three patented, broad-spectrum, synthetic polymer anti-infectives: RECCE® 327 as an intravenous and topical therapy that is being developed for the treatment of serious and potentially life-threatening infections due to Gram-positive and Gram-negative bacteria including their superbug forms; RECCE® 435 as an orally administered therapy for bacterial infections; and RECCE® 529 for viral infections. Through their multi-layered mechanisms of action, Recce’s anti-infectives have the potential to overcome the hypercellular mutation of bacteria and viruses – the challenge of all existing antibiotics to date.
The FDA has awarded RECCE® 327 Qualified Infectious Disease Product designation under the Generating Antibiotic Initiatives Now (GAIN) Act – labelling it for Fast Track Designation, plus 10 years of market exclusivity post approval. Further to this designation, RECCE® 327 has been included on The Pew Charitable Trusts Global New Antibiotics in Development Pipeline as the world’s only synthetic polymer and sepsis drug candidate in development. RECCE® 327 is not yet market approved for use in humans with further clinical testing required to fully evaluate safety and efficacy.
Recce wholly owns its automated manufacturing, which is supporting present clinical trials. Recce’s anti-infective pipeline seeks to exploit the unique capabilities of its technologies targeting synergistic, unmet medical needs.
Corporate Contact
James Graham
Recce Pharmaceuticals Ltd
+61 (02) 9256 2571
James.graham@recce.com.au
Media & Investor Relations (AU)
Andrew Geddes
CityPR
+61 (02) 9267 4511
ageddes@citypublicrelations.com.au
Media (USA)
Jordyn Temperato
LifeSci Communications
jtemperato@lifescicomms.com
Investor Relations (USA & EU)
Guillame van Renterghem
LifeSci Advisors
gvanrenterghem@lifesciadvisors.com
1 https://www.tga.gov.au/sites/default/files/special-access-scheme-guidance-for-health-practitioners-and-sponsors.pdf
2 https://www.ncbi.nlm.nih.gov/books/NBK482221/
3 https://www.cdc.gov/mrsa/index.html#:~:text=Methicillin%2Dresistant%20Staphylococcus%20aureus%20(MRSA),-Related%20Pages&text=Staph%20bacteria%20are%20usually%20harmless,of%20resistance%20to%20some%20antibiotics.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/4fe46bec-dbbf-498b-8a4a-961938bc1842
https://www.globenewswire.com/NewsRoom/AttachmentNg/14b01c08-494d-4e4d-9386-f905d959f19b
https://www.globenewswire.com/NewsRoom/AttachmentNg/c960736d-5e91-408c-8503-8c8d54c867b5
https://www.globenewswire.com/NewsRoom/AttachmentNg/7c3197fb-d441-43ed-bc67-2489c62d1489