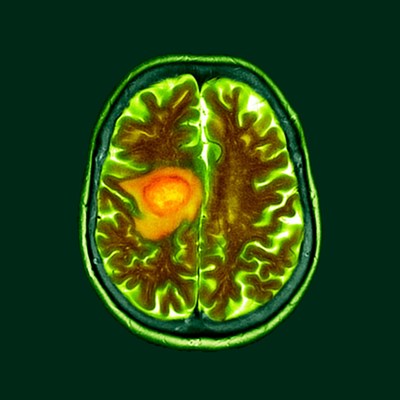
Recombinetics Inc., a leading gene editing company with platform technology applied to biomedicine and animal agriculture, today announced that it has partnered with American Preclinical Services in a first of its kind study to improve Glioblastoma outcomes.
|
EAGAN, Minn., Oct. 19, 2020 /PRNewswire/ -- Recombinetics Inc., a leading gene editing company with platform technology applied to biomedicine and animal agriculture, today announced that it has partnered with American Preclinical Services in a first of its kind study to improve Glioblastoma outcomes. Surrogen, a subsidiary of Recombinetics Inc., is focused on developing large animal models of human disease for use in medical research. In September, Surrogen displayed the world's first genetically engineered large animal model of Glioblastoma, which develops in a predictable and reproducible timeframe. This important scientific advancement was achieved through somatic cell gene editing to engineer the precise genetic changes seen in human tumors. Glioblastoma, or GBM, is a devastating disease which results in a cancerous tumor that develops in the brain. GBM is the most common and aggressive of all brain tumors, with few effective treatment options available, and a five-year survival rate of less than 5%. One of the most significant challenges for the development of new therapies or treatments is the lack of reliable and reproducible animal models of the disease for use in pre-clinical studies. With this breakthrough, that is no longer the case. This technology presents a unique opportunity to impact the lives of those diagnosed with Glioblastoma and to give families hope. According to Dr. Adrienne Watson, Recombinetics' Vice President of Research and Development, "This new model of glioblastoma gives us the opportunity to better understand this disease and improve patient outcomes by identifying tumor biomarkers, developing new surgical and imaging technologies, and most importantly, creating new therapeutic techniques to treat these tumors." "The goal is to improve the therapeutic development landscape by being able to reproduce the effects of therapies effective in animals, in human patients, as well as improve dose selection prior to entering clinical trials. Overall, the technology may speed up the process of bringing viable therapies to market," says CEO, Mark Platt. "One of the most difficult tasks in preclinical research today is developing models of human disease in animals that can then be dependably used to test the effectiveness of novel methods for treatment. These new large-animal disease models will introduce a new paradigm for evaluating new medical devices, new surgical techniques, new drugs or drug delivery systems with substantially higher degree of translatability to clinical outcomes. These models have the potential to reduce both the development times for new therapies and the risk of failure of a new modality in very expensive clinical trials," states Jim Pomonis, CSO, American Preclinical Services. About Recombinetics Contact: Nikki Rockstroh, (612) 727-2000, 257023@email4pr.com About American Preclinical Services Contact: Michael Frie, 257023@email4pr.com
SOURCE Recombinetics Inc |