Regenerex Pharma, Inc., announces the establishment of its comprehensive management team and the commencement of the Company manufacturing plans in Q4 2023.
2024 Growth Strategy and Product Launch to Reset the Wound Bed Space and Accelerate the Healing Process in Chronic Wounds with 95% Success Rate
LAS VEGAS--(BUSINESS WIRE)-- Regenerex Pharma, Inc. (OTC: RGPX), announces the establishment of its comprehensive management team and the commencement of the Company manufacturing plans in Q4 2023. Regenerex Pharma’s products heal wounds effectively with its proprietary innovative formulas in the shortest period of time to minimize the chances of future complications. The products have shown to be very effective in healing chronic wounds in multiple clinical evaluations with 95% of wounds demonstrating 100% closure within 90 days. All of our products feature our proprietary QBx™ ingredients which contribute to setting up a suitable environment to allow wounds to close.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230928693287/en/
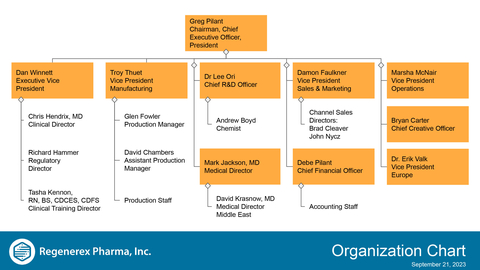
Regenerex Pharma, Inc. Key Management Officers and Directors Organization Chart (Graphic: Business Wire)
Currently, there are no products available on the market that are successful in healing chronic, non-healing wounds through the down regulation of proteases. Other modern wound dressings such as hydrocolloids and collagens absorb wound fluids, but these dressings do not impact the cellular environment with simple gauze and gauze-like dressings to cover and protect the wound. The Company will be manufacturing its complete line of wound care products from its facility in Memphis, TN. Company products include the following:
1. Xcellderma™- OTC- Liquid Bandage Skin Protectant
Xcellderma™ products are sterile wound dressings and are effective for treating diabetic foot ulcers, pressure ulcers, and other chronic wounds. During the last several years, a scientific and medical consensus has emerged that elevated protease levels impede wound healing. QBx™ the active ingredient down regulates the production of certain proteases and matrix metalloproteases, or MMPs, which are protein enzymes that are proven to impede the healing of a majority of chronic wounds. Approximately 80% of chronic wounds display elevated levels of proteases (including MMPs).
2. Accelerex- Sterile Wound Cream
The first commercially available medical device, Accelerex, is for the treatment of a wide variety of chronic and acute wounds. Accelerex is a custom-designed, FDA and CE approved unit-dose, sterile wound dressing impregnated with an ointment containing QBx. Chronic wounds are generally defined as wounds that have not healed after thirty days of consistent clinical treatment, and include diabetic ulcers, burns, pressure ulcers (bedsores), and venous stasis ulcers. The Company’s broadly-enabling technology was discovered from oak bark extract and referred to as QBx™.
3. Accelerex- Impregnated Sterile Wound Dressing
For use as a wound dressing to manage pressure ulcers (stages I-IV), stasis ulcers, diabetic skin ulcers, skin irritations, cuts, and abrasions. FDA-cleared, prescription-only combination device that blends the benefits of a wound dressing with two drug components. Provides 3 modes of action to help treat acute and chronic wounds: Protective dressing, moisturizing ointment and 2 drug components: rubidium chloride and potassium chloride.
About Regenerex Pharma, Inc.
Regenerex Pharma, Inc. provides the most technologically advanced clinically proven products on the market today that successfully heal wounds quicker, conveniently and cost effectively. Currently, there are no products available on the market that are successful in healing chronic, non-healing wounds through the down regulation of proteases. Other modern wound dressings such as hydrocolloids and collagens absorb wound fluids, but these dressings do not impact the cellular environment with simple gauze and gauze-like dressings to cover and protect the wound.
We manufacture and market the most efficacious, clinically proven advanced chronic wound care product line in the world. Wounds that don’t heal detract from your quality of life and prevent you from doing things you enjoy. Regenerex’s products provide advanced wound care that can stimulate healing effectively and in shorter period of time compared to traditional methods. Our QBx™ technology contributes to setting up a suitable environment to allow wounds to close. More information about the Company may be found at regenerexpharmainc.com
Notice regarding forward-looking statements
This press release contains forward-looking statements that may be subject to various risks and uncertainties. Such forward-looking statements are made pursuant to the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 and may include statements regarding our future financial performance or results of operations, including expected revenue growth. Unless otherwise required by law, we undertake no obligation to publicly update or revise any forward looking statements, whether as a result of new information, future events or otherwise after the date of this press release. Additional information concerning risks and uncertainties that would cause actual results to differ materially from those projected or suggested in the forward-looking statements can be found in the reports that we have filed with the Securities and Exchange Commission.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230928693287/en/
Contacts
Regenerex Pharma, Inc.
Investor Relations Ph: (305) 927-5191
Company Ph: 877-761-RGPX (7479)
Email: investors@regenerexpharmainc.com
regenerexpharmainc.com
Source: Regenerex Pharma, Inc.
Regenerex Pharma, Inc. Key Management Officers and Directors Organization Chart (Graphic: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20230928693287/en







