Respiratory Syncytial Virus Market Outlook 2024-2034:
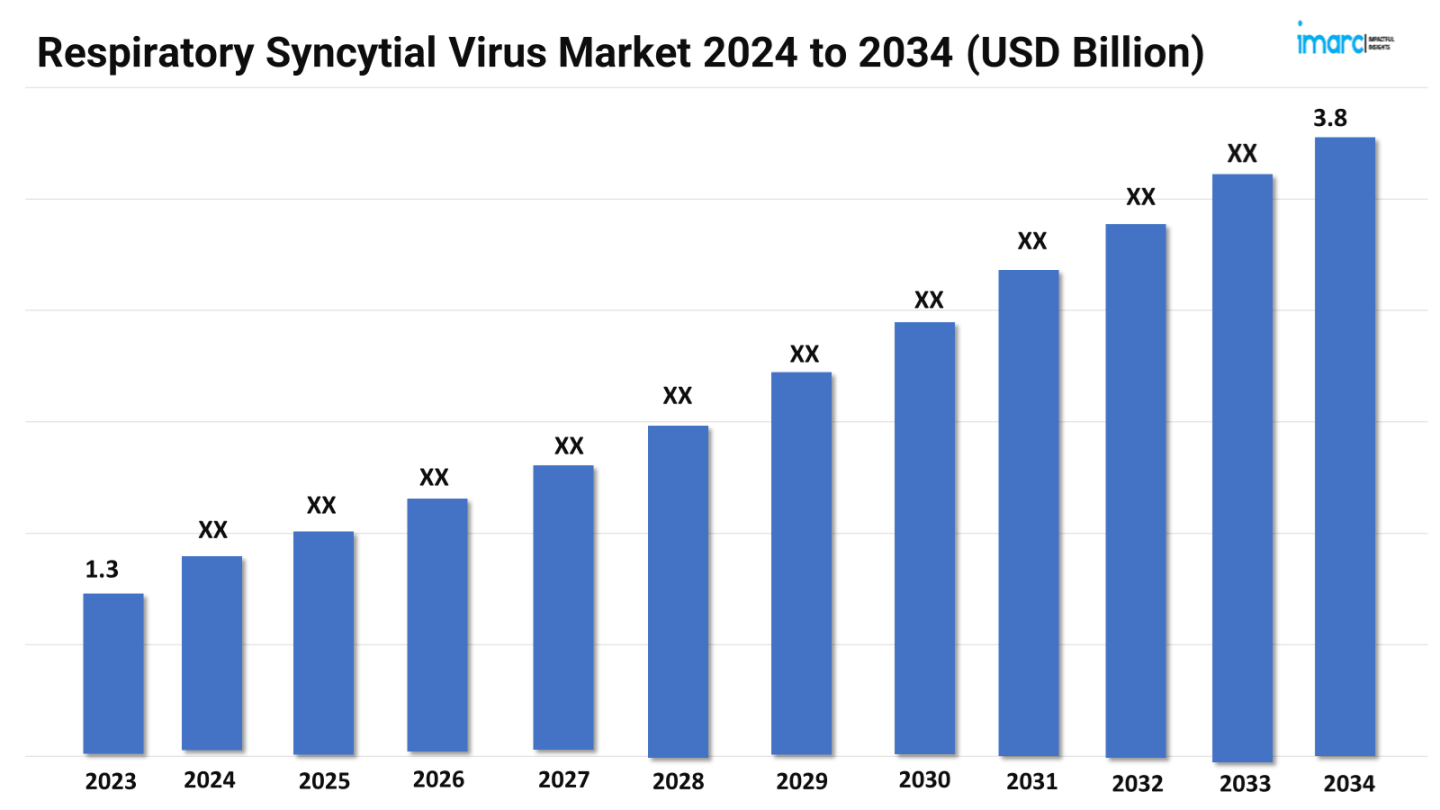
The respiratory syncytial virus market size reached a value of US$ 1.3 Billion in 2023. Looking forward, the market is expected to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 10.01% during 2024-2034. The market is experiencing rapid growth due to heightened focus on vaccine development and novel therapeutics. Increased incidence in vulnerable populations, such as infants and the elderly, is spurring innovation in prevention and treatment. Advances in monoclonal antibodies and antiviral drugs are driving market expansion.
Advancements in Vaccines: Driving the Respiratory Syncytial Virus Market
Advancements in vaccines for the respiratory syncytial virus (RSV) market are transforming the landscape of respiratory infection prevention, particularly for vulnerable populations such as infants, the elderly, and individuals with compromised immune systems. A major trend is the progress in developing vaccines that offer broad protection across various RSV strains. Researchers are focusing on creating vaccines that stimulate a robust immune response, including both humoral and cellular immunity. One notable advancement is the development of prefusion F protein-based vaccines, which have shown promise in preclinical and clinical trials. This approach targets the RSV fusion protein in its prefusion state, which is critical for the virus's entry into host cells, thereby enhancing vaccine efficacy. Additionally, the incorporation of adjuvants to boost immune responses and the use of novel delivery platforms, such as mRNA technology, are making waves in RSV vaccine research. mRNA vaccines, similar to those used for COVID-19, offer rapid development and adaptability to emerging RSV strains, potentially accelerating the availability of effective vaccines.
Request a PDF Sample Report: https://www.imarcgroup.com/respiratory-syncytial-virus-market/requestsample
Another significant advancement is the focus on maternal vaccination. Vaccinating pregnant women can provide passive immunity to newborns, offering protection during their most vulnerable early months of life. Clinical trials are underway to assess the safety and efficacy of such vaccines in pregnant populations. Overall, these advancements are expected to reduce the burden of RSV-related illnesses significantly, offering hope for improved public health outcomes and a decrease in healthcare costs associated with severe RSV infections. The continued investment in research and development is crucial to bringing these innovative vaccines to market and ensuring widespread access.
Innovations in Therapeutics: Contributing to Market Expansion
Innovations in therapeutics for the respiratory syncytial virus market are revolutionizing the treatment landscape, aiming to address the significant health burden posed by severe RSV infections. One of the key innovations is the development of monoclonal antibodies, such as palivizumab and the newer long-acting monoclonal antibodies. These therapies are designed to provide passive immunity by targeting the RSV fusion protein, preventing the virus from entering and infecting cells. Newer formulations offer extended duration of action, reducing the need for frequent dosing and improving patient compliance, particularly in high-risk populations like premature infants and elderly individuals. Another notable advancement is the progress in antiviral drug development. Several novel antiviral agents are undergoing clinical trials, aiming to inhibit RSV replication and reduce the severity and duration of symptoms. For example, drugs targeting specific viral enzymes or proteins essential for RSV replication are showing promise in early studies. These antiviral therapies are anticipated to complement existing treatments and offer more precise and effective options for managing RSV infections.
Additionally, there is growing interest in combination therapies that integrate antiviral drugs with existing treatments to enhance efficacy. These combinations aim to address multiple stages of the viral life cycle or synergize with other therapeutic modalities, potentially leading to more comprehensive treatment regimens. The development of inhaled therapies is also gaining traction. These therapies deliver drugs directly to the respiratory tract, providing targeted treatment with potentially fewer systemic side effects. Inhaled antiviral agents and anti-inflammatory drugs are being explored to alleviate symptoms and improve outcomes in severe RSV cases. Overall, these innovations are setting the stage for more effective and targeted management of RSV, promising to improve patient outcomes and reduce the overall burden of RSV-related illnesses. The ongoing research and development in this field are crucial for advancing therapeutic options and meeting the needs of diverse patient populations.
Increased Awareness and Diagnostics:
Increased awareness and advancements in diagnostics are significantly transforming the respiratory syncytial virus market, enhancing both early detection and management of RSV infections. Enhanced public and healthcare professional awareness of RSV has led to better recognition of symptoms and more timely medical interventions. Educational campaigns and guidelines emphasize the importance of early diagnosis, particularly in high-risk groups. This increased awareness is driving higher rates of testing and diagnosis, helping to reduce the impact of severe RSV infections by initiating early and appropriate treatment. Innovations in diagnostic technologies are also playing a pivotal role in this transformation. The development of rapid, accurate, and accessible diagnostic tests is crucial for managing RSV effectively. Advances include polymerase chain reaction (PCR) assays and molecular tests that provide rapid results with high sensitivity and specificity. These tests enable healthcare providers to diagnose RSV infections more quickly and accurately, facilitating timely treatment and isolation measures to prevent the spread of the virus.
Additionally, point-of-care diagnostic devices are emerging, allowing for on-site testing in various healthcare settings, including primary care and emergency departments. These devices offer the advantage of immediate results, which is particularly valuable in urgent care situations and can lead to quicker decision-making and intervention. The integration of diagnostic advancements with electronic health records (EHR) and data analytics is further enhancing disease surveillance and management. By tracking RSV cases and outcomes, healthcare systems can better understand infection patterns, optimize resource allocation, and implement targeted public health strategies. Overall, increased awareness and advancements in diagnostics are crucial in managing RSV more effectively. They contribute to improved patient outcomes, reduced transmission rates, and a better understanding of the virus, ultimately leading to more effective public health responses and treatment strategies.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8904&method=587
Leading Companies in the Respiratory Syncytial Virus Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global respiratory syncytial virus market, several notable companies are creating vaccines that offer broad protection, particularly for high-risk groups such as infants and the elderly. This trend reflects a growing commitment to preventing RSV-related hospitalizations and reducing the overall burden of the disease. Pfizer Inc. and Meissa Vaccines have been investing heavily in their manufacturing capacities in recent months.
Pfizer Inc. announced in Hong Kong the availability of its bivalent RSV prefusion F (RSVpreF) vaccine. The vaccine helps fight against lower respiratory tract disease (LRTD) caused by RSV in adults aged 60 years and older, as well as LRTD and severe LRTD caused by RSV in newborns from birth up to six months of age by active immunization of pregnant women.
Meissa Vaccines announced favorable results from a clinical trial of the intranasal live attenuated MV-012-968 vaccine against respiratory syncytial virus in individuals aged six to 36 months. The trial evaluated the safety and immunogenicity of the needle-free, adjuvant-free MV-012-968 vaccine candidate in RSV-naive seronegative subjects.
Apart from this, Blue Lake Biotechnology, Inc. reported preliminary results from the first two cohorts of a Phase 1/2a clinical trial for BLB201, the company's investigational vaccine against severe respiratory syncytial virus disease. The data suggest that BLB201 is immunogenic and well tolerated, with no serious safety issues recorded after a single intranasal dose in RSV seropositive children aged 18 to 59 months. The continuing experiment is now recruiting both RSV seropositive and RSV seronegative children as young as eight months old.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8904&flag=E
Regional Analysis:
The major markets for the respiratory syncytial virus include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for respiratory syncytial virus while also representing the biggest market for its treatment. This can be attributed to the integration of digital health technologies, including telemedicine and health monitoring apps.
Moreover, there is a trend towards the development and adoption of more advanced diagnostic tools for RSV. Rapid and accurate diagnostic tests, including molecular assays and point-of-care devices, are becoming more widely available. These innovations facilitate early detection, timely treatment, and better management of RSV infections.
Apart from this, the market is increasingly targeting high-risk populations, such as premature infants, elderly individuals, and those with underlying health conditions. Public health initiatives and healthcare strategies emphasize the need for preventive measures and specialized treatments for these vulnerable groups.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the respiratory syncytial virus market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the respiratory syncytial virus market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current respiratory syncytial virus marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/respiratory-syncytial-virus-market
IMARC Group Offer Other Reports:
Lambert-Eaton Myasthenic Syndrome Market: The 7 major lambert-eaton myasthenic syndrome market is expected to exhibit a CAGR of 5.15% during the forecast period from 2024 to 2034.
Germ Cell Tumor Market: The 7 major germ cell tumor market reached a value of US$ 1.5 Billion in 2023, and projected the 7MM to reach US$ 3.7 Billion by 2034, exhibiting a growth rate (CAGR) of 8.91% during the forecast period from 2024 to 2034.
Scedosporium Infection Market: The 7 majors scedosporium infection market is expected to exhibit a CAGR of 5.8% during the forecast period from 2024 to 2034.
Genital Herpes Market: The 7 majors genital herpes market is expected to exhibit a CAGR of 3.49% during the forecast period from 2024 to 2034.
Gastroesophageal Reflux Disease Market: The 7 majors gastroesophageal reflux disease market is expected to exhibit a CAGR of 1.21% during the forecast period from 2024 to 2034.
Trauma Market: The 7 majors trauma market is expected to exhibit a CAGR of 5.78% during the forecast period from 2024 to 2034.
Seasonal Influenza Market: The 7 major seasonal influenza market reached a value of US$ 8.9 Billion in 2023, and projected the 7MM to reach US$ 37,213.7 Million by 2034, exhibiting a growth rate (CAGR) of 15.08% during the forecast period from 2024 to 2034.
Short Bowel Syndrome Market: The 7 major short bowel syndrome market is expected to exhibit a CAGR of 20.52% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The respiratory syncytial virus market size reached a value of US$ 1.3 Billion in 2023. Looking forward, the market is expected to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 10.01% during 2024-2034. The market is experiencing rapid growth due to heightened focus on vaccine development and novel therapeutics. Increased incidence in vulnerable populations, such as infants and the elderly, is spurring innovation in prevention and treatment. Advances in monoclonal antibodies and antiviral drugs are driving market expansion.
Advancements in Vaccines: Driving the Respiratory Syncytial Virus Market
Advancements in vaccines for the respiratory syncytial virus (RSV) market are transforming the landscape of respiratory infection prevention, particularly for vulnerable populations such as infants, the elderly, and individuals with compromised immune systems. A major trend is the progress in developing vaccines that offer broad protection across various RSV strains. Researchers are focusing on creating vaccines that stimulate a robust immune response, including both humoral and cellular immunity. One notable advancement is the development of prefusion F protein-based vaccines, which have shown promise in preclinical and clinical trials. This approach targets the RSV fusion protein in its prefusion state, which is critical for the virus's entry into host cells, thereby enhancing vaccine efficacy. Additionally, the incorporation of adjuvants to boost immune responses and the use of novel delivery platforms, such as mRNA technology, are making waves in RSV vaccine research. mRNA vaccines, similar to those used for COVID-19, offer rapid development and adaptability to emerging RSV strains, potentially accelerating the availability of effective vaccines.
Request a PDF Sample Report: https://www.imarcgroup.com/respiratory-syncytial-virus-market/requestsample
Another significant advancement is the focus on maternal vaccination. Vaccinating pregnant women can provide passive immunity to newborns, offering protection during their most vulnerable early months of life. Clinical trials are underway to assess the safety and efficacy of such vaccines in pregnant populations. Overall, these advancements are expected to reduce the burden of RSV-related illnesses significantly, offering hope for improved public health outcomes and a decrease in healthcare costs associated with severe RSV infections. The continued investment in research and development is crucial to bringing these innovative vaccines to market and ensuring widespread access.
Innovations in Therapeutics: Contributing to Market Expansion
Innovations in therapeutics for the respiratory syncytial virus market are revolutionizing the treatment landscape, aiming to address the significant health burden posed by severe RSV infections. One of the key innovations is the development of monoclonal antibodies, such as palivizumab and the newer long-acting monoclonal antibodies. These therapies are designed to provide passive immunity by targeting the RSV fusion protein, preventing the virus from entering and infecting cells. Newer formulations offer extended duration of action, reducing the need for frequent dosing and improving patient compliance, particularly in high-risk populations like premature infants and elderly individuals. Another notable advancement is the progress in antiviral drug development. Several novel antiviral agents are undergoing clinical trials, aiming to inhibit RSV replication and reduce the severity and duration of symptoms. For example, drugs targeting specific viral enzymes or proteins essential for RSV replication are showing promise in early studies. These antiviral therapies are anticipated to complement existing treatments and offer more precise and effective options for managing RSV infections.
Additionally, there is growing interest in combination therapies that integrate antiviral drugs with existing treatments to enhance efficacy. These combinations aim to address multiple stages of the viral life cycle or synergize with other therapeutic modalities, potentially leading to more comprehensive treatment regimens. The development of inhaled therapies is also gaining traction. These therapies deliver drugs directly to the respiratory tract, providing targeted treatment with potentially fewer systemic side effects. Inhaled antiviral agents and anti-inflammatory drugs are being explored to alleviate symptoms and improve outcomes in severe RSV cases. Overall, these innovations are setting the stage for more effective and targeted management of RSV, promising to improve patient outcomes and reduce the overall burden of RSV-related illnesses. The ongoing research and development in this field are crucial for advancing therapeutic options and meeting the needs of diverse patient populations.
Increased Awareness and Diagnostics:
Increased awareness and advancements in diagnostics are significantly transforming the respiratory syncytial virus market, enhancing both early detection and management of RSV infections. Enhanced public and healthcare professional awareness of RSV has led to better recognition of symptoms and more timely medical interventions. Educational campaigns and guidelines emphasize the importance of early diagnosis, particularly in high-risk groups. This increased awareness is driving higher rates of testing and diagnosis, helping to reduce the impact of severe RSV infections by initiating early and appropriate treatment. Innovations in diagnostic technologies are also playing a pivotal role in this transformation. The development of rapid, accurate, and accessible diagnostic tests is crucial for managing RSV effectively. Advances include polymerase chain reaction (PCR) assays and molecular tests that provide rapid results with high sensitivity and specificity. These tests enable healthcare providers to diagnose RSV infections more quickly and accurately, facilitating timely treatment and isolation measures to prevent the spread of the virus.
Additionally, point-of-care diagnostic devices are emerging, allowing for on-site testing in various healthcare settings, including primary care and emergency departments. These devices offer the advantage of immediate results, which is particularly valuable in urgent care situations and can lead to quicker decision-making and intervention. The integration of diagnostic advancements with electronic health records (EHR) and data analytics is further enhancing disease surveillance and management. By tracking RSV cases and outcomes, healthcare systems can better understand infection patterns, optimize resource allocation, and implement targeted public health strategies. Overall, increased awareness and advancements in diagnostics are crucial in managing RSV more effectively. They contribute to improved patient outcomes, reduced transmission rates, and a better understanding of the virus, ultimately leading to more effective public health responses and treatment strategies.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8904&method=587
Leading Companies in the Respiratory Syncytial Virus Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global respiratory syncytial virus market, several notable companies are creating vaccines that offer broad protection, particularly for high-risk groups such as infants and the elderly. This trend reflects a growing commitment to preventing RSV-related hospitalizations and reducing the overall burden of the disease. Pfizer Inc. and Meissa Vaccines have been investing heavily in their manufacturing capacities in recent months.
Pfizer Inc. announced in Hong Kong the availability of its bivalent RSV prefusion F (RSVpreF) vaccine. The vaccine helps fight against lower respiratory tract disease (LRTD) caused by RSV in adults aged 60 years and older, as well as LRTD and severe LRTD caused by RSV in newborns from birth up to six months of age by active immunization of pregnant women.
Meissa Vaccines announced favorable results from a clinical trial of the intranasal live attenuated MV-012-968 vaccine against respiratory syncytial virus in individuals aged six to 36 months. The trial evaluated the safety and immunogenicity of the needle-free, adjuvant-free MV-012-968 vaccine candidate in RSV-naive seronegative subjects.
Apart from this, Blue Lake Biotechnology, Inc. reported preliminary results from the first two cohorts of a Phase 1/2a clinical trial for BLB201, the company's investigational vaccine against severe respiratory syncytial virus disease. The data suggest that BLB201 is immunogenic and well tolerated, with no serious safety issues recorded after a single intranasal dose in RSV seropositive children aged 18 to 59 months. The continuing experiment is now recruiting both RSV seropositive and RSV seronegative children as young as eight months old.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8904&flag=E
Regional Analysis:
The major markets for the respiratory syncytial virus include the United States, Germany, France, the United Kingdom, Italy, Spain and Japan. According to projections by IMARC, the United States has the largest patient pool for respiratory syncytial virus while also representing the biggest market for its treatment. This can be attributed to the integration of digital health technologies, including telemedicine and health monitoring apps.
Moreover, there is a trend towards the development and adoption of more advanced diagnostic tools for RSV. Rapid and accurate diagnostic tests, including molecular assays and point-of-care devices, are becoming more widely available. These innovations facilitate early detection, timely treatment, and better management of RSV infections.
Apart from this, the market is increasingly targeting high-risk populations, such as premature infants, elderly individuals, and those with underlying health conditions. Public health initiatives and healthcare strategies emphasize the need for preventive measures and specialized treatments for these vulnerable groups.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the respiratory syncytial virus market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the respiratory syncytial virus market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current respiratory syncytial virus marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/respiratory-syncytial-virus-market
IMARC Group Offer Other Reports:
Lambert-Eaton Myasthenic Syndrome Market: The 7 major lambert-eaton myasthenic syndrome market is expected to exhibit a CAGR of 5.15% during the forecast period from 2024 to 2034.
Germ Cell Tumor Market: The 7 major germ cell tumor market reached a value of US$ 1.5 Billion in 2023, and projected the 7MM to reach US$ 3.7 Billion by 2034, exhibiting a growth rate (CAGR) of 8.91% during the forecast period from 2024 to 2034.
Scedosporium Infection Market: The 7 majors scedosporium infection market is expected to exhibit a CAGR of 5.8% during the forecast period from 2024 to 2034.
Genital Herpes Market: The 7 majors genital herpes market is expected to exhibit a CAGR of 3.49% during the forecast period from 2024 to 2034.
Gastroesophageal Reflux Disease Market: The 7 majors gastroesophageal reflux disease market is expected to exhibit a CAGR of 1.21% during the forecast period from 2024 to 2034.
Trauma Market: The 7 majors trauma market is expected to exhibit a CAGR of 5.78% during the forecast period from 2024 to 2034.
Seasonal Influenza Market: The 7 major seasonal influenza market reached a value of US$ 8.9 Billion in 2023, and projected the 7MM to reach US$ 37,213.7 Million by 2034, exhibiting a growth rate (CAGR) of 15.08% during the forecast period from 2024 to 2034.
Short Bowel Syndrome Market: The 7 major short bowel syndrome market is expected to exhibit a CAGR of 20.52% during the forecast period from 2024 to 2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800