SAB Biotherapeutics, Inc. (Nasdaq:SABS), (“SAB” or “the company”) today announced its financial results for the fourth quarter of 2023 in addition to its full year financial results for the fiscal year ended December 31, 2023, and reported on recent accomplishments and expected upcoming milestones.
Sioux Falls, SD , March 29, 2024 (GLOBE NEWSWIRE) -- SAB Biotherapeutics Reports Full Year 2023 Operating and Financial Results
March 29, 2024
SAB-142 Phase 1 trial on track for data release during 2024
Completed financing for up to $110 million with leading life science investors
Cash and equivalents of $56.6 million as of December 31, 2023
Company expects its cash and equivalents, with exercise of Tranche B warrants, will fund operations into 2026
Sioux Falls, SD March 29, 2024 (GlobeNewswire)—SAB Biotherapeutics, Inc. (Nasdaq:SABS), (“SAB” or “the company”), a clinical-stage biopharmaceutical company with a novel immunotherapy platform that is developing human anti-thymocyte immunoglobulin (hIgG) for delaying the onset or progression of Type 1 diabetes (T1D), today announced its financial results for the fourth quarter of 2023 in addition to its full year financial results for the fiscal year ended December 31, 2023, and reported on recent accomplishments and expected upcoming milestones.
“During 2023, we significantly changed the direction of our company to focus on SAB-142, our innovative, human anti-thymocyte immune globulin for the prevention of progression of T1D” stated Samuel J. Reich, Chairman and CEO of SAB. “Our pivot to SAB-142 catalyzed our capital raise of up to $110 million, led by a dedicated group of specialist investors, many of whom have deep knowledge about the needs of patients with T1D. We are grateful for their support in our drive to bring the benefits of SAB-142 to T1D patients in need. Despite recent advances in therapy, there remains a meaningful void for therapies that can prevent the progression of this devastating illness. We remain on track to provide Phase 1 data for SAB-142 by the end of 2024.”
Highlights from 4Q23 and Recent Corporate Developments
- Closed an equity financing for up to $110 million with leading life sciences investors, generating funds sufficient for operations into 2026 assuming the exercise of all outstanding Tranche B Warrants.
- Initiated a Phase 1 clinical trial of SAB-142 for safety and tolerability in autoimmune disorders including T1D.
- Announced Michael G. King Jr. as new Chief Financial Officer. Mr. King has extensive experience and prior success as an award-winning biotechnology research analyst and senior advisor with more than 25 years of experience with investors, banking institutions and thought leaders in various pharmaceutical disciplines.
- Appointed Andrew Moin, Partner and Analyst at Sessa Capital, a New York based investment advisor registered with the SEC, to the SAB Board of Directors. Mr. Moin has been with Sessa since 2012, where he works on idea generation, research, and investment implementation. He has also been deeply involved in the type 1 diabetes community for over 20 years, including as a volunteer and member of the Young Leadership Committee of the New York City Chapter of the JDRF and an early supporter of multiple fundamental diabetes research and innovation projects.
- Appointed Katie Ellias, Managing Partner at JDRF T1D Fund, to the SAB Board of Directors. The JDRF T1D Fund LLC is a venture philanthropy fund with approximately $200 million in assets, including an investment in SAB. Ms. Ellias joined the T1D Fund in 2018 where she has led several investments in companies developing T1D-oriented therapies. She has also served as a director on the board of several companies, including, DiogenX, Veralox Therapeutics, i2O Therapeutics, and Capillary Biomedical.
- Welcomed Erick Lucera to our Board of Directors. Mr. Lucera, a 30-year veteran of the biotechnology and medical device industry, has held executive positions at several healthcare companies, most recently as Chief Financial Officer of Editas Medicine.
- Hosted 2023 R&D Day virtual and in-person events, affirming SAB’s strategic focus in the autoimmunity space with SAB-142, a disease-modifying fully human hIgG aimed at preventing onset or disease progression of Type 1 Diabetes, and subsequently can be expanded into other immunology indications.
Upcoming Corporate Presentation
Samuel J. Reich, Chairman and CEO of SAB Biotherapeutics, will present on behalf of the company at the upcoming 23rd Annual Needham Virtual Conference on Thursday, April 11, 2024, at 1:30 pm ET. The company will also participate in virtual 1:1 meetings. A replay of Mr. Reich’s presentation will be archived on the SAB Biotherapeutics website for up to one year.
Fiscal Year 2023 Financial Results
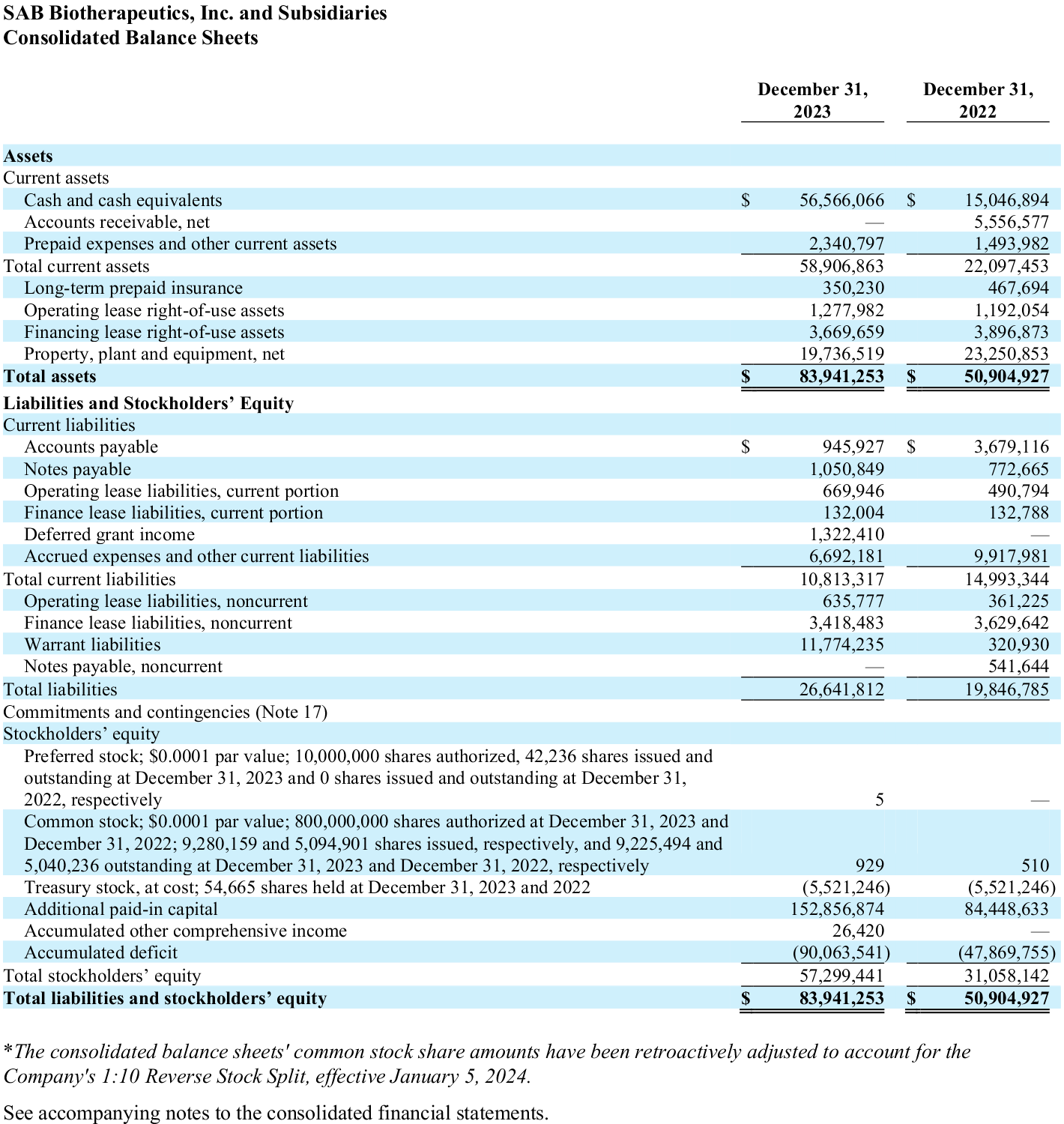
- SAB Biotherapeutics held cash and equivalents of $56.6 million at December 31, 2023, compared to $15.0 million at December 31, 2022.
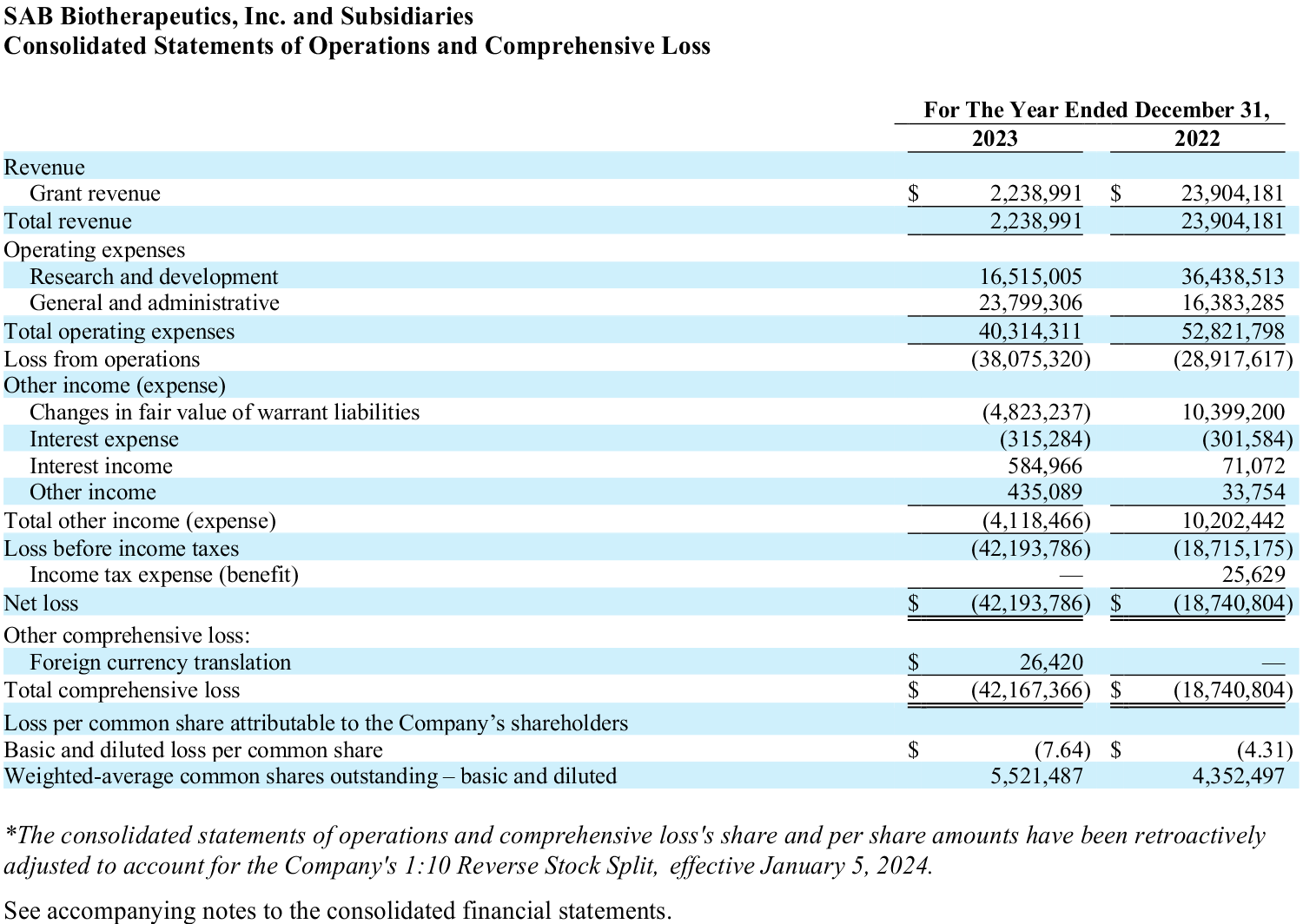
- R&D expenses of $16.5 million and $36.4 million for the years ended December 31, 2023 and December 31, 2022 respectively.
- General and administrative expenses of $23.8 million and $16.4 million for the years ended December 31, 2023 and December 31, 2022 respectively.
- Other net expense of $4.1 million for the year ended December 31, 2023, and net other income of $10.2 million for the year ended December 31, 2022.
- SAB reported a net loss of $42.2 million and $18.7 million for the years ended December 31, 2023 and December 31, 2022 respectively.
2024 Cash Guidance
The company believes it has sufficient current and future cash via the exercise of all outstanding Tranche B warrants sufficient to fund its operations into 2026.
About SAB Biotherapeutics, Inc.
SAB Biotherapeutics (SAB) is a clinical-stage biopharmaceutical company focused on developing fully human, multi- targeted, high-potency immunoglobulins (IgGs), without the need for human donors or convalescent plasma, to treat and prevent immune and autoimmune disorders. The Company’s lead asset, SAB-142, targets type 1 diabetes (T1D) with a disease-modifying therapeutic approach that aims to change the treatment paradigm by delaying onset and potentially preventing disease progression. Using advanced genetic engineering and antibody science to develop Transchromosomic (Tc) Bovine™, the only transgenic animal with a human artificial chromosome, SAB’s DiversitAb™ drug development production system is able to generate a diverse repertoire of specifically targeted, high-potency, fully-human IgGs that can address a wide range of serious unmet needs in human diseases without the need for convalescent plasma or human donors. For more information on SAB, visit: https://www.SAb.bio/ and follow SAB on Twitter and LinkedIn.
Forward-Looking Statements
Certain statements made herein that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “believe,” “may,” “will,” “to be,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “should,” “would,” “plan,” “predict,” “potential,” “seem,” “seek,” “future,” “outlook,” and similar expressions that predict or indicate future events or trends or that are not statements of historical matters. These forward-looking statements include, but are not limited to, statements regarding future events, including the development and efficacy of our T1D program, and other discovery programs, the funding of the tranche B warrants issued in the Company’s private placement offering, financial projections and future financial and operating results (including estimated cost savings and cash runway), the outcome of and potential future government, and other third-party collaborations or funded programs.
These statements are based on the current expectations of SAB and are not predictions of actual performance, and are not intended to serve as, and must not be relied on, by any investor as a guarantee, prediction, definitive statement, or an assurance, of fact or probability. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties and other factors which may be beyond our control. Actual events and circumstances are difficult or impossible to predict, and these risks and uncertainties may cause our or our industry’s results, performance, or achievements to be materially different from those anticipated by these forward-looking statements. A further description of risks and uncertainties can be found in the sections captioned “Risk Factors” in our most recent annual report on Form 10-K, as amended, subsequent quarterly reports on Form 10-Q, as may be amended or supplemented from time to time, and other filings with or submissions to, the U.S. Securities and Exchange Commission, which are available at https://www.sec.gov/. Except as otherwise required by law, SAB disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events, or circumstances or otherwise.
Contacts
Media Relations: khollon@sab.bio
Investor Relations: matt@milestone-advisorsllc.com

Media Relations: khollon@sab.bio Investor Relations: matt@milestone-advisorsllc.com





