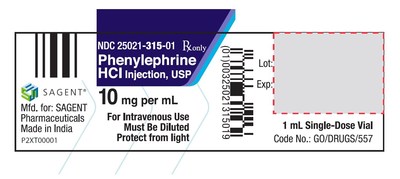
Sagent Pharmaceuticals, Inc. today announced the voluntary nationwide recall of three lots of Phenylephrine Hydrochloride Injection, USP (10 mg/mL)
| SCHAUMBURG, Ill., March 11, 2021 /PRNewswire/ -- Sagent Pharmaceuticals, Inc. today announced the voluntary nationwide recall of three lots of Phenylephrine Hydrochloride Injection, USP (10 mg/mL). This product was manufactured by Indoco Remedies Ltd. and distributed by Sagent Pharmaceuticals, Inc. Sagent has initiated this voluntary recall of Phenylephrine Hydrochloride Injection, USP to the user level as the result of a customer complaint due to potentially loose crimped vial overseals. A non-integral crimped vial overseal may result in a non-sterile product. Intravenous administration of a product intended to be sterile that is not sterile could result in serious systemic infections which may be life-threatening. The possibility of a breach in sterility assurance in distributed product, while remote, cannot be eliminated. To date, Sagent has not received reports of any adverse events associated with this issue. Phenylephrine Hydrochloride Injection, USP is an alpha-1 adrenergic receptor agonist indicated for the treatment of clinically important low blood pressure resulting primarily from the dilation of blood vessels, which decreases blood pressure in the setting of anesthesia. The product is supplied in 3 mL glass tubular vials. The lot numbers being recalled were distributed to hospitals, wholesalers and distributors nationwide in the USA from 11/17/2020 – 03/08/2021.
Customers are being notified by fax, email, FedEx, and/or certified mail that includes arrangements for return of all recalled product. Customers have been instructed to examine their inventory immediately and to quarantine, discontinue distribution of and return the recalled lots listed above. Customers who may have further distributed this product have been requested to identify their customers and notify them at once of this product recall. Consumers/distributors/retailers that have product which is being recalled should stop using product and return the recalled product. The necessary form by which to document this information as well as other information regarding this recall is available at www.Sagentpharma.com. Customers or consumers with any questions about returning unused product should be directed to the customer call center at (866) 625-1618 M-F, 8am-7pm CST. Healthcare workers who have medical questions about Phenylephrine Hydrochloride Injection, USP, may contact Medical Affairs (866-625-1618, Option 3) M-F, 8am-5pm CST. Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this product. Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration. CUSTOMER SUPPORT: MEDICAL AFFAIRS
SOURCE Sagent Pharmaceuticals |