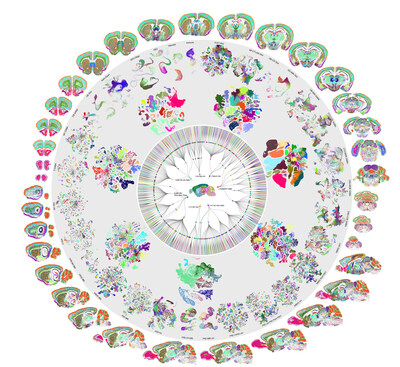
Six years and 32 million cells later, scientists have created the first full cellular map of a mammalian brain.
High-resolution atlas charts neural neighborhoods for more than 5,300 cell types
SEATTLE, Dec. 13, 2023 /PRNewswire/ -- Six years and 32 million cells later, scientists have created the first full cellular map of a mammalian brain.
In a set of 10 papers in Nature today, a network of researchers unveiled an atlas cataloging the location and type of every cell in the adult mouse brain. Using advanced technologies that profile individual cells, the teams identified over 5,300 cell types – far more than known before – and pinpointed their locations within the brain's intricate geography.
"Having a complete 'parts list' of the brain will help accelerate efforts to unravel how it works," said Hongkui Zeng, Ph.D., Executive Vice President and Director of the Allen Institute for Brain Science.
"This is a landmark achievement that really opens the door for the next stage of investigations of the brain's function, development and evolution, akin to the reference genomes for studying gene function and genomic evolution," said Zeng, who led one of the studies. "My colleagues said that the 5,000 cell types we identified will keep neuroscientists busy for the next 20 years trying to figure out what these cell types do and how they change in disease."
The collective work is a capstone for the National Institutes of Health's BRAIN Initiative Cell Census Network, or BICCN. Hundreds of researchers contributed to the project, which was funded by the NIH's Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, or The BRAIN Initiative®.
"Where we previously stood in darkness, this milestone achievement shines a bright light, giving researchers access to the location, function, and pathways between cell types and cell groups in a way we couldn't imagine previously," said John Ngai, Ph.D., Director of the NIH BRAIN Initiative. "This product is a testament to the power of this unprecedented, cross-cutting collaboration and paves our path for more precision brain treatments."
Location, location, location
By combining single-cell RNA sequencing with spatial transcriptomics—methods for determining which genes are expressed in individual cells and where those cells are located—Zeng and her collaborators revealed the brain’s astonishing complexity and diversity.
One of the atlas's major revelations is the deep connection between a cell's genetic identity and its spatial position, Zeng said. This relationship underscores how location shapes function, offering clues into the evolutionary history and intricate interactions of different brain regions.
"We're seeing the building blocks of the brain's circuits," she said. "The brain's organization likely reflects its evolutionary history."
One intriguing finding is the distinct cellular organization between the lower ("ventral") versus the upper ("dorsal") parts of the brain. While the ancient ventral part features a mosaic of interrelated cells, the more recent dorsal part contains fewer but highly divergent cell types. This distinction could be a key to deciphering how different brain regions evolved unique roles, for example, the ventral part for basic survival and the dorsal part for adaptation, Zeng said.
The researchers also found that transcription factors – proteins that regulate gene activity – comprise a 'code' that specifies a cell's identity.
The atlas also uncovered how brain cells talk to each other via a diverse cast of signaling molecules, which carry messages from cell to cell. That diversity enables complex interactions between different cell types.
The strong alignment across independently collected genomic, epigenomic, and spatial datasets provides high confidence that this atlas maps more than just cell identities – it captures the true organizational blueprints underlying mammalian brain development, Zeng said.
Looking ahead, the atlas can serve as a model for similar mappings in the brains of other species—namely our own. That work is already underway.
It also provides a guide to genetically target specific cell types, enabling tools to study specific functions and disease. This could pave the way for precision treatments, Zeng said.
"We know that many diseases originate in specific parts of the brain, and probably in specific cell types," she said. "With this map in hand, we can gain a more precise view of the dysfunction of disease and then create genetic or pharmacologic tools to target those specific cell types, to achieve greater efficacy and minimal side effects."
Allen Institute scientists also co-led a study to create a detailed map of the neurons that connect the brain to the spinal cord, enabling movement and sensory modulation. In this study, a team led by Zhigang He, Ph.D., and Carla Winter, M.D., Ph.D., of Harvard provides the most in-depth characterization of these spinal-projecting neurons (SPNs) to date. By integrating the molecular identities and locations of these neurons into one atlas, scientists gain insight into how this intricate network controls function and movement. "And by having a baseline map of these cell types, we can now study how spinal cord injuries or stroke alter them and hopefully develop targeted therapies," said Dr. Winter.
Allen Institute scientists contributed to five other studies, including:
- A spatial atlas of cell types in the whole mouse brain. In this study, led by Xiaowei Zhuang, Ph.D., of Harvard, scientists used spatially resolved transcriptomic profiling of >1,100 genes to reveal the spatial organization of >5,000 transcriptionally distinct clusters across the entire mouse brain. Registration of the cell atlas to the Allen Common Coordinate Framework allows quantification of cell type composition and organization in each brain region. The high-resolution spatial map reveals cell-cell interactions and molecular underpinnings between hundreds of cell-type pairs.
- A comparison of gene regulatory programs across different species, including humans. In this study, researchers analyzed certain regions of DNA that act like switches, turning genes on or off and controlling a cell's identity. The team found that so-called jumping genes—DNA sequences that can flit about the genome—make up the bulk of human-specific "switches" in the neocortex. As these same regions can also be involved in neurodegenerative diseases, further study could point the way to new therapies, the authors said. "This data is a gold mine for geneticists who can now start to uncover the molecular basis of complex traits like schizophrenia," said Bing Ren, Ph.D., of UCSD, who co-led the study with the Salk Institute's Joseph Ecker, Ph.D.
About the Allen Institute
The Allen Institute is an independent, 501(c)(3) nonprofit research organization founded by philanthropist and visionary, the late Paul G. Allen. The Allen Institute is dedicated to answering some of the biggest questions in bioscience and accelerating research worldwide. The Institute is a recognized leader in large-scale research with a commitment to an open science model. Its research institutes and programs include the Allen Institute for Brain Science, launched in 2003; the Allen Institute for Cell Science, launched in 2014; the Allen Institute for Immunology, launched in 2018; and the Allen Institute for Neural Dynamics, launched in 2021. In 2016, the Allen Institute expanded its reach with the launch of The Paul G. Allen Frontiers Group, which identifies pioneers with new ideas to expand the boundaries of knowledge and make the world better. For more information, visit alleninstitute.org.
Media Contact
Peter Kim, Sr. Manager, Media Relations
206-605-9884 | peter.kim@alleninstitute.org
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/scientists-unveil-first-complete-cellular-map-of-adult-mouse-brain-302013647.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/scientists-unveil-first-complete-cellular-map-of-adult-mouse-brain-302013647.html
SOURCE Allen Institute