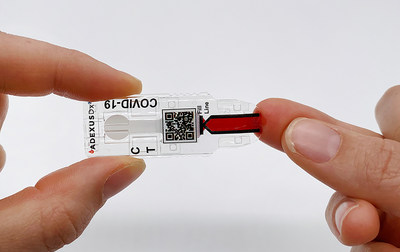
NOWDiagnostics, Inc. announced today that it has received Conformité Européene (CE) mark approval for its ADEXUSDx® COVID-19 antibody test for use in moderate/complex laboratory settings across 28 countries in the European Union (EU)
|
SPRINGDALE, Ark., July 28, 2020 /PRNewswire/ -- NOWDiagnostics, Inc. announced today that it has received Conformité Européene (CE) mark approval for its ADEXUSDx® COVID-19 antibody test for use in moderate/complex laboratory settings across 28 countries in the European Union (EU). The application for CE marking of the ADEXUSDx® COVID-19 antibody test was submitted mid-June. With a CE marking, C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics, will begin offering the ADEXUSDx® COVID-19 Test for use in a variety of health care settings in the EU—from clinics to hospital emergency rooms, while launching clinical trials of the test for use at point-of-care and over-the-counter. The ADEXUSDx® COVID-19 Test is a rapid serology, self-contained assay that measures the presence of SARS-CoV-2 antibodies to deliver accurate and reliable results in 15 minutes with no buffers, reagents, or additional equipment. The ADEXUSDx® COVID-19 Test has demonstrated a high level of analytical performance with 95.6% sensitivity (true positive rate) and 98.5% specificity (true negative rate). NOWDiagnostics submitted an application to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the test on May 29, 2020. The application is still pending. "The ADEXUSDx® COVID-19 Test is literally a lab at the tip of your finger, specifically designed to make diagnostic testing possible anywhere. While this CE mark permits our test to be used in laboratory settings, we expect that after completing clinical trials and obtaining further regulatory approvals, our ADEXUSDx® COVID-19 Test will be available to everyone. CE mark of our test is the first step in making that a reality," said Kevin Clark, Chief Executive Officer of NOWDiagnostics, Inc. The ADEXUSDx® COVID-19 Test uses an FDA-cleared and CE-marked platform containing next-generation, easy-to-use technology that is affordable, portable, and delivers laboratory-quality results in minutes without any additional supplies. The test is developed and manufactured at NOWDiagnostics' Springdale, Arkansas facility with materials sourced from American suppliers and uses a drop (40 μL) of whole blood, plasma, or serum to detect the presence of antibodies to SARS-CoV-2, the virus that causes COVID-19. This can include those who have been infected with the virus, regardless of whether they presented with severe, moderate, mild or no symptoms, as well as those who have cleared infection. Early in the U.S. COVID-19 crisis, when hospitals were overwhelmed with COVID-19 patient care challenges and laboratories were scrambling to access and validate new COVID-19 tests, NOWDiagnostics intentionally partnered with reputable laboratories nationwide to collect hundreds of well characterized samples and to screen all possible combinations of proteins to deliver a best-in-class diagnostic test for the fight against COVID-19. Employing sophisticated separation microfluidics and proprietary multi-layer membranes, the ADEXUSDx® COVID-19 Test separates plasma from whole blood, automatically controlling the sample volume allowing for highly accurate antibody testing and, in minutes, displays results indicating whether the subject has developed SARS-CoV-2 antibodies for COVID-19. The Centers for Disease Control and Prevention (CDC) and others are analyzing data from antibody tests to estimate the number of people who have been infected with COVID-19 in the U.S., to learn more about how the body's immune system responds to the virus, and to explore how the virus spreads. This information will also be critical for the development of a vaccine. We believe that the features of the self-contained ADEXUSDx® COVID-19 Test, from its simplicity to its portability, make it uniquely suited for large-scale and remote testing situations, as well as under resourced areas lacking access to traditional health care laboratories and facilities, like those encountered by those fighting on the frontline, in particular, healthcare employees, police, fire, emergency medical services and military personnel. NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits and limitations, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.
SOURCE NOWDiagnostics |