Soterix Medical Inc. (SMI), a global leader in non-invasive stimulation and synergistic brain monitoring technologies, announced today that patient recruitment for its treatment resistant depression trial funded by National Institute of Health has begun in New York City.
|
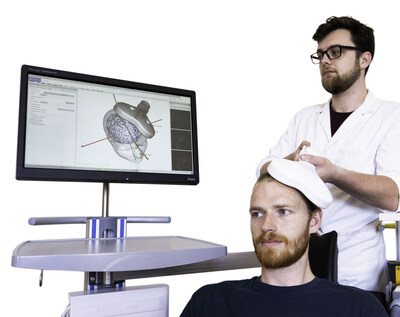
WOODBRIDGE, N.J., May 8, 2023 /PRNewswire/ -- Soterix Medical Inc. (SMI), a global leader in non-invasive stimulation and synergistic brain monitoring technologies, announced today that patient recruitment for its treatment resistant depression trial funded by National Institute of Health has begun in New York City. For the trial, SMI is actively enrolling individuals who received but did not adequately respond to repetitive Transcranial Magnetic Stimulation (rTMS). Each trial site is responsible for reviewing prospective patients to determine if enrollment criteria is met by the individual. Soterix Medical Announces Patient Recruitment for Parcel-Guided rTMS Depression Trial
SMI with technology licensed from Columbia University is developing a cloud-based targeting software based on parcel-guided rTMS (pg-rTMS). The pg-rTMS approach leverages anatomical magnetic-resonance-imaging (MRI), machine-learning (ML), and data from the Human Connectome Project (HCP) atlas, to design optimized personalized rTMS therapy. Dr. Abhishek Datta, CEO and CTO of Soterix Medical explains, "Parcel-guided rTMS represents a major technical advance in rTMS therapy by combining three unique SMI technologies. One, Soterix Medical provides the unique FDA-cleared navigation system, not interrupted by line-of-site issues. Two, Soterix Medical has developed seamless cloud-based systems for neuromodulation optimization. And three, the TMS targeting approach developed by Columbia University provides the key to delivering rTMS therapy optimized to each patient's brain-circuit." While conventional rTMS over the left dorsolateral prefrontal cortex is currently an FDA-approved treatment for treatment-refractory depression, it remains only partially effective with response and remission rates of ~41% and ~35% respectively. In a previous pilot study, pg-rTMS targeting a specific parcel of the HCP atlas led to a 100% treatment response in patients who were resistant to the conventional rTMS therapy. Dr. Dennis Truong, Scientist at Soterix Medical and Principal Investigator of the project says, "We are excited for this next stage in the validation of parcel-guided rTMS therapy for depression. This trial is intended to confirm that personalized TMS delivery is crucial to deliver the fullest therapeutic value of TMS, to all patients, even those that did not respond adequately to conventional rTMS." Prospective participants should contact Soterix Medical immediately at contact@soterixmedical.com to determine eligibility / obtain additional information.
SOURCE Soterix Medical, Inc. |