A new study marks a significant advance in developing a gene therapy for X-linked retinitis pigmentosa, a hereditary disease that leads to severe sight loss in young males.
MIAMI, Feb. 24, 2020 /PRNewswire/ -- A new study marks a significant advance in developing a gene therapy for X-linked retinitis pigmentosa, a hereditary disease that leads to severe sight loss in young males.
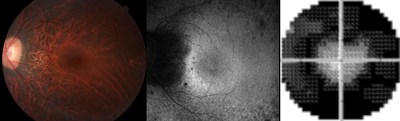
Researchers at Bascom Palmer Eye Institute, the Department of Ophthalmology at the University of Miami Miller School of Medicine, took part in the international multi-center study and are actively participating in further clinical trials. It's the latest published study for the Bascom Palmer specialists who have performed nearly 100 gene therapy surgeries to date for several types of inherited retinal disorders.
"X-linked retinitis pigmentosa related to the GTPase regulator (RPGR) gene is one of the most common and severe types of this disease," said Byron L. Lam, M.D., the Robert Z. & Nancy J. Greene Professor of Ophthalmology, and Bascom Palmer's principal investigator. "This gene therapy study offers hope for patients with this currently untreatable blinding disease."
The study, "Initial Results from a First-in-Human Gene Therapy Trial on X-linked Retinitis Pigmentosa Caused by Mutations in RPGR," was published February 24 in the journal Nature Medicine. Bascom Palmer co-authors with Dr. Lam were Janet L Davis, M.D., Leach Distinguished Professor of Ophthalmology; Ninel Gregori, M.D., professor of clinical ophthalmology; and Potyra Rosa, M.D., clinical coordinator. Dr. Robert E. MacLaren of Oxford University led the study on this novel treatment. In addition, Dr. René Moya at the University of Chile, and Dr. Juliana Sallum at Federal University of São Paulo, Brazil, provided support by referring patients for the study.
A total of 18 patients took part in the six-month Phase I/II dose escalation clinical trial for X-linked retinitis pigmentosa (RP) caused by the RPGR gene mutation, which blocks production of a protein necessary for proper functioning of the photoreceptor cells in the retina. The mutation accounts for approximately 70 percent of all cases of X-linked retinitis pigmentosa, which causes night blindness in early childhood followed by progressive daytime vision loss.
"The results from this initial trial showed no significant safety concerns after gene therapy surgery," said Dr. Davis. "Visual field improvements were observed in some patients, and these favorable findings support Bascom Palmer's wide-ranging gene therapy research."
Since performing RPGR gene therapy surgery using an adeno-associated viral vector on one patient in the initial study, Dr. Davis and Dr. Gregori have treated nine more patients using the optimized dosing determined from the initial trial. Those results have not yet been published.
"The initial phase I/II study provides the basis for an ongoing randomized phase II/III clinical trial," said Dr. Lam. "Bascom Palmer is the only U.S. site participating in these trials. We now have more than 15 patients enrolled in the new study, and our team is forging ahead with gene therapy research in many types of inherited retinal diseases."
In 2018, Bascom Palmer ophthalmologists performed one of the nation's first gene therapy surgeries using Luxturna, the first ocular gene therapy drug approved by the Food and Drug Administration, to treat a child with a blinding vision disease. "We have treated a significant number of those cases since then," said Dr. Gregori.
Last year, Drs. Lam, Davis and Gregori published results of a phase II clinical trial of gene therapy for choroideremia, another inherited disorder that causes progressive vision loss in males. "We are now taking part in a phase III trial for this treatment," said Dr. Davis.
Dr. Gregori said most patients in Bascom Palmer's gene therapy trials are men in their 20s and 30s who are experiencing serious vision problems. Patients are given genetic tests to identify the mutation so that appropriate gene therapy can be administered.
"We use advanced microperimetry technology to identify the cells that are still functioning and deliver the new genes into those cells in the retina," she said.
The Bascom Palmer team has deep experience in delivering the appropriate localized dose to the patient's eye, added Dr. Gregori. "It's very exciting to see the impact our gene therapy surgeries can make in the quality of vision of our patients."

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/study-advances-development-of-gene-therapy-for-x-linked-retinitis-pigmentosa-301010157.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/study-advances-development-of-gene-therapy-for-x-linked-retinitis-pigmentosa-301010157.html
SOURCE Bascom Palmer Eye Institute