Sumitomo Heavy Industries, Ltd. announced the decision to invest in Alpha Fusion Inc., a developer of Astatine based radiopharmaceuticals for Targeted Alpha Therapy.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230308005372/en/
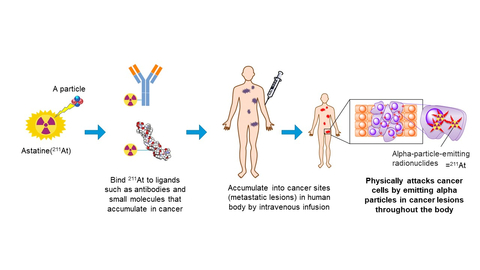
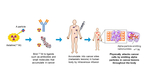
Image Diagram of TAT (Graphic: Business Wire)
AF will aim at establishing their pipeline (*1). Development of their radiopharmaceuticals is expected to be progressed by this funding and will lead more needs of Astatine-211. SHI is joining a new project led by Osaka University for mass production of Astatine-211 and will contribute for the development of the TAT, which is expected to be a future advanced medicine, with our technology of particle accelerators and a synthesis of radio-isotope (RI) labeled compounds.
TAT and Astatine-211
TAT is a treatment that destroys cancer cells in the human body by injecting alpha emitting RI labeled candidate which selectively targets to cancer cells. Astatine-211 (211At, Atomic number 85, Half life time 7.2H) is an element belonging to halogens and has the property of emitting alpha particle. Due to no stable isotopes in nature, their properties have not been fully elucidated so far. However, it's getting attention in accordance with recent development of a nuclear medicine treatment and/or a theranostics (a new term combinated of therapy and diagnostic). Clinical studies had been already conducted in U.S. and Sweden etc. because of the advantages which is different from other approved RIs (177Lu, 223Ra, or 131I). The advantages are; stable production using naturally abundant bismuth (209Bi) with a relatively low-energy particle accelerator (cyclotron), short half-life time as 7.2 hours, and direct chemical formation with basic structure of candidates which conjugate with the disease target site.
In Japan, investigator-initiated clinical trials targeting differentiated thyroid cancer (jRCT2051210144) at Osaka University and pheochromocytoma paraganglioma (jRCT2021220012) at Fukushima Medical University have started and further progress is highly expected.
Alpha Fusion Inc.
AF is a startup company who is conducting a social implementation of Astatine-211 based on drug discovery transferred research results of Osaka university funded by Program on Open Innovation Platform with Enterprises, Research Institute and Achademia (OPERA) promoted by Japan Science and Technology Agency (JST). In the field of targeted alpha therapy, which has been rapidly attracting worldwide attention in recent years, Mission of the company is to let the Astatine drug development out by using its originality from Japan in the field of TAT that is rapidly attracting worldwide attention. AF aims to lead this innovative modality into the basic of cancer treatment and are aiming at the practical application of new cancer treatment by proceeding with pipeline research and development to business development on a world-class level.
|
Company Profile |
||
|
Alpha Fusion Inc. |
Head office |
2-5-13, Umeda, Kita-ku, Osaka, Japan |
|
Board of director |
CEO, Sunao Fujioka |
|
|
Business domain |
Technical research, drug discovery development, and business development of astatine-introduced compounds into a wide range of small molecules and antibodies, including Sodium astatide |
|
|
Capital |
251 MJPY |
|
|
Web site |
||
(*1) Pipelines of AF are followings. Please refer to the URL for detail.
• Pipeline 1 : Drug for thyroid cancer
(Under Phase I clinical trial : https://prtimes.jp/main/html/rd/p/000000001.000091191.html)
• Pipeline 2 : Drug for prostate cancer
(Preparing for clinical trial in next year : https://prtimes.jp/main/html/rd/p/000000003.000091191.html)
• Other 4 drugs are under development and preparing for a patent application for the basic technology
View source version on businesswire.com: https://www.businesswire.com/news/home/20230308005372/en/
Sumitomo Heavy Industries, Ltd., Corporate Communication Department, +81-3-6737-2332
Alpha Fusion Inc., info@alpha-fusion.com
Source: Sumitomo Heavy Industries, Ltd.
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20230308005372/en