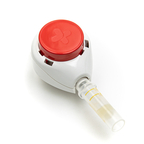
Tasso, Inc. today announced that the TassoOne™ Plus, a new high-volume liquid blood collection device, has received CE Mark certification and successfully fulfilled all of the performance, safety, and relevant product requirements under the new European Union Medical Device Regulation.
SEATTLE--(BUSINESS WIRE)-- Tasso Inc., the leading provider of patient-centric, clinical-grade blood collection solutions, today announced that the TassoOne™ Plus, a new high-volume liquid blood collection device, has received CE Mark certification and successfully fulfilled all of the performance, safety, and relevant product requirements under the new European Union Medical Device Regulation. With this CE Mark, Tasso now expands its capabilities to offer patient-centric, high-volume blood collection solutions for the European market.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230214005224/en/
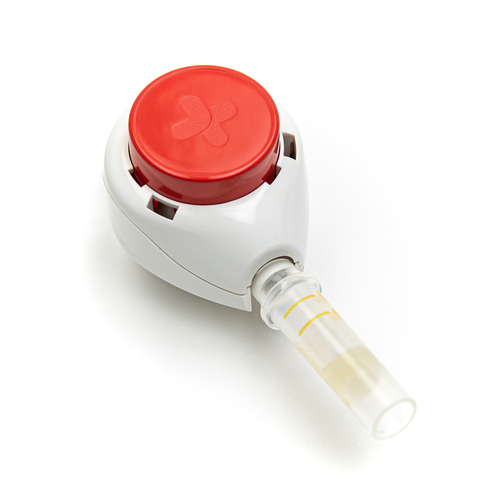
The TassoOne™ Plus is Tasso's latest high-volume liquid blood collection device for decentralized clinical trials and home diagnostic testing initiatives throughout Europe. (Photo: Business Wire)
The TassoOne Plus is the latest addition to Tasso’s signature line of CE Marked blood collection solutions for dried and liquid samples. Users collect their own blood through an easy, virtually painless process that can be completed at home. The process generates higher volumes and superior sample quality compared to traditional remote blood sampling techniques. The clinical-grade samples fit seamlessly into existing downstream analysis workflows, empowering pharmaceutical companies, healthcare organizations, and academic institutions across the European Economic Area.
“The demand for convenient, patient-centric care is exploding, and Tasso is on a mission to bring high-quality healthcare into homes worldwide,” said Ben Casavant, Ph.D., CEO and co-founder of Tasso. “This CE Mark unlocks clinical-grade liquid blood collection for decentralized clinical trials and home healthcare within the European Union, accelerating and expanding access to care. Regulatory clearances like this one are a testament to the quality and safety of our products.”
For more information about Tasso’s high-volume liquid blood collection devices, please visit http://www.tassoinc.com/high-volume.
About Tasso
Tasso is an emerging healthcare technology company that is transforming the traditional blood collection paradigm with a patient-centric approach. The company’s devices enable simple, convenient, and virtually painless blood collection for users. Tasso technology has the power to bring healthcare anywhere, any time. Headquartered in Seattle, Washington, Tasso is privately held and funded by grants, investments, and co-development deals with various industry leaders. For more information, please visit www.tassoinc.com.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230214005224/en/
Contacts
Commercial Contact
RJ Asplund
(818) 434-5004
rasplund@tassoinc.com
Media Contact
Dina Schneider
(206) 822-4186 x1016
media@tassoinc.com
Source: Tasso, Inc.