TheraCell Inc., a leading allograft solution company, today announced that it has completed the first two spine surgical cases using the TheraFuze DBF® Fiber Bag™.
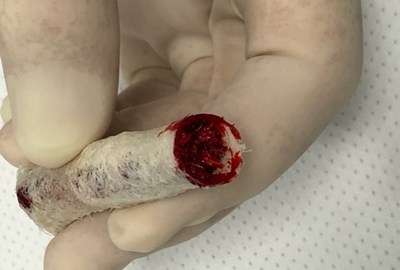
| LOS ANGELES, April 6, 2021 /PRNewswire/ -- TheraCell Inc., a leading allograft solution company, today announced that it has completed the first two spine surgical cases using the TheraFuze DBF® Fiber Bag™. The Fiber Bag is an allograft alternative to the resorbable polymer mesh bag products that are currently on the market. It is made from 100% demineralized cortical bone fibers generated with the company’s patented manufacturing process and formed using its proprietary FiberLok™ technology. This allows the Fiber Bag to maintain its form and integrity after filling and rehydration, permitting manipulation and implantation by the surgeon.
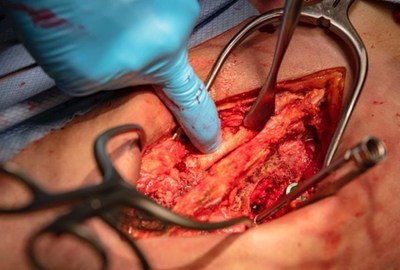
“The first true all biologic solution where the containment bag actually participates in the fusion process” One of the cases was performed by Dr. Frank Phillips, The Ronald DeWald, Endowed Professor of Spinal Deformities, Director of the Division of Spine Surgery and Section Head of Minimally Invasive Spine Surgery at Rush University Medical Center. The case was a multi-level posterior lumbar decompression and fusion. Dr. Phillips packed the Fiber Bags with autograft and laid the fully contained constructs in the posterolateral gutters. “The Fiber Bag is the best graft containment solution I have ever used and the only one that is actually a significant graft in itself. It’s a true all biologic solution where the containment bag actually participates in the fusion process,” said Dr. Phillips. The second case was completed by Dr. Jack Zigler, Orthopedic Spine Surgeon at the Texas Back Institute and Co-Director of the Center for Disc Replacement. Dr. Zigler rehydrated the Fiber Bags with aspirated blood from the vertebral body and placed them in the intertransverse gutters. “I was impressed with how quickly and easily the Fiber Bags absorbed the aspirate. The grafts swelled and filled out with gelatinous clot, yet maintained their structure throughout the procedure. Great product and great handling characteristics!” stated Dr. Zigler. This next generation posterolateral solution is designed to be an improved alternative to both traditional boats and resorbable polymer mesh bags, offering complete containment of graft material within a bag that is itself osteoconductive and osteoinductive. The Fiber Bag may be packed with additional graft of the surgeon’s choosing, and may be sutured for closure following rehydration. The fully contained fiber bag construct may be placed in the posterolateral gutters, in a bone void or cavity, or may be delivered using minimally invasive techniques. The Fiber Bag is part of TheraCell’s comprehensive portfolio of TheraFuze DBF procedure-specific graft solutions, currently available in the United States directly through TheraCell. About TheraCell, Inc. TheraCell is focused on the development of advanced technologies for bone and soft tissue repair and the inventor of the next-generation TheraFuze DBF® demineralized bone fiber technology. It is also in the process of bringing its novel O2ssify™ oxygenation technology to market that has been demonstrated to supercharge bone formation. Founded in 2008, the firm is headquartered in Los Angeles, CA and maintains offices and laboratories in Littleton, MA. www.theracellinc.com. TheraFuze DBF®, TheraFuze DBF® Fiber Bag™, FiberLok™, and The Bag That Is A Graft™ are trademarks of TheraCell, Inc. For more information on these products, please visit TheraCell’s website.
SOURCE TheraCell, Inc. |