TheraCell Inc., a leading allograft solution company, announced that over 200 of its proprietary Fiber Anchors have been implanted.
TheraCell has surpassed 200 Fiber Anchors implanted, providing surgeons a solution for improved screw fixation
LOS ANGELES, July 6, 2021 /PRNewswire/ -- TheraCell Inc., a leading allograft solution company, today announced that over 200 of its proprietary Fiber Anchors have been implanted. The limited commercial release of this unique product has demonstrated that use doubled quarter over quarter and reflects repeat usage by multiple surgeons in more than a dozen locations.
Dr. Frank Phillips, The Ronald DeWald, Endowed Professor of Spinal Deformities, Director of the Division of Spine Surgery and Section Head of Minimally Invasive Spine Surgery at Rush University Medical Center, has utilized the TheraFuze DBF Fiber Anchor in several cases and states, "The Fiber Anchor from TheraCell is one of the most unique and effective biologic products I have ever used. This technology adds immense value in revision surgery as well as in longer deformity constructs where loss of hardware fixation and resultant failure of fusion is a significant concern".
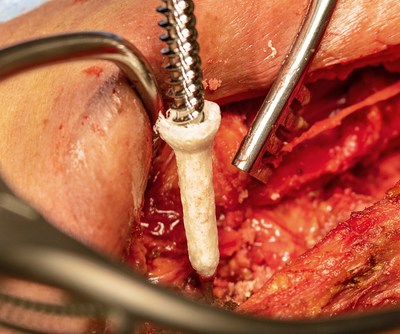
The TheraFuze DBF Fiber Anchor is a novel product that acts much like a drywall anchor, providing immediate improvement in screw fixation in primary surgeries and revision cases. It is made from 100% demineralized cortical bone fibers generated with the company's patented manufacturing process. Testing has demonstrated that it more than doubles the pullout force versus screws implanted without the Fiber Anchor. TheraFuze DBF Fiber Anchors are sized to be used with 5.5-6.0 mm, 6.5-7.0 mm, 7.5-8.0 mm and 8.5-9.0 mm screws.
"This is a significant milestone for TheraCell," said Bradley Patt, PhD, TheraCell's President and CEO. "We are proud to offer this highly differentiated clinical solution and we are very pleased with the success of our limited launch."
The TheraFuze DBF Fiber Anchor is part of TheraCell's comprehensive portfolio of TheraFuze DBF procedure-specific graft solutions, including the TheraFuze DBF Fiber Bag™ and TheraFuze DBF Fiber Wrap™ for graft containment, TheraFuze DBF Fiber Bullets™ for delivery through a cannula in MIS cases, and 1, 2, and 3-level TheraFuze DBF Fiber Boats™.
About TheraCell, Inc.
TheraCell is focused on the development of advanced technologies for bone and soft tissue repair and the inventor of the next-generation TheraFuze DBF® demineralized bone fiber technology. It is also in the process of bringing its novel O2ssify™ oxygenation technology to market that has been demonstrated to supercharge bone formation. Founded in 2008, the firm is headquartered in Los Angeles, CA and maintains offices and laboratories in Littleton, MA. www.theracellinc.com. TheraFuze DBF®, Fiber Anchor™, Fiber Bullet™, Fiber Bag™, Fiber Wrap™ and Fiber Boat™ are trademarks of TheraCell, Inc.
For more information on these products, please visit TheraCell's website.
|
Media Contact: |
Product Information: |
|
Bradley Patt, PhD TheraCell, Inc. 818-645-4081 |
Curt Cooper TheraCell, Inc. 630-953-9594 |
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/theracell-exceeds-expectations-with-its-limited-launch-of-the-therafuze-dbf-fiber-anchor-301325689.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/theracell-exceeds-expectations-with-its-limited-launch-of-the-therafuze-dbf-fiber-anchor-301325689.html
SOURCE TheraCell, Inc.