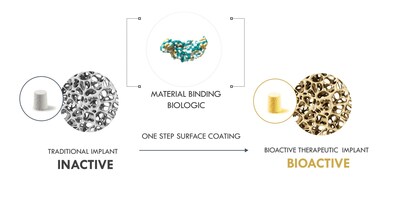
This week, Theradaptive, a leading biotechnology company specializing in therapeutic delivery platforms, presents a new abstract demonstrating the efficacy and safety of the company’s novel AMP2 protein technology in rabbit PLF and sheep interbody fusion models.
FREDRICK, Md., Feb. 9, 2023 /PRNewswire/ -- This week, Theradaptive, a leading biotechnology company specializing in therapeutic delivery platforms, presents a new abstract demonstrating the efficacy and safety of the company's novel AMP2 protein technology in rabbit PLF and sheep interbody fusion models. At the Orthopaedic Research Society's annual meeting in Dallas this week, the company will demonstrate how coating an implant with its proprietary material-binding osteoinductive AMP2 protein resulted in precise localization of bone formation and consistent spinal fusion without off-target effects. Researchers observed 100% fusion compared to the 60% associated with current autograft technologies in PLF.
The latest alternatives to autograft involve incorporating the recombinant osteoinductive protein rhBMP-2, but this approach has been associated with adverse events like inflammation and bone growth in unwanted areas. By re-engineering rhBMP-2 to create a material-binding protein variant AMP2, Theradaptive has created a protein that induces bone-growth more potently and in a more precisely localized way than rhBMP-2, thus vastly reducing off-target effects. The Theradaptive process binds AMP2 to ReBOSSIS, a 510K-approved implant material, to create its OsteoAdapt SP Spinal Fusion implant, a safer alternative to commercially available rhBMP-2.
In this study, scientists from Theradaptive and the University of Iowa sought to assess the ability of OsteoAdapt SP to induce spinal fusion in rabbit posterolateral and sheep interbody models. 12 rabbits underwent single level posterolateral fusion, using OsteoAdapt SP rather than autograft. Three sheep underwent two-level lumbar interbody fusion using high or low dose OsteoAdapt SP. In the rabbit PLF model, OsteoAdapt SP demonstrated an impressive 100% fusion, compared to the 60% associated with iliac crest bone grafts, the current best practice. In the pilot sheep interbody model, CTs showed increase in density of new bone formation over time with evidence of bridging bone by 4-8 weeks at the high dose and 8-12 weeks at the low dose.
CEO and founder of Theradaptive, Dr. Luis Alvarez, Ph.D. sees these results as an encouraging step towards human clinical trials: 'Current autograft treatments are painful, they risk adverse events, and they only work around 60% of the time. Seeing OsteoAdapt SP beat the current standard of care in these well accepted models so convincingly in preclinical trials gives us optimism that targeted regenerative technology like AMP2 will improve outcomes for patients who currently have few options. We expect to start first in human trials later this year.'
Douglas Fredericks, Director of the University of Iowa Bone Healing Research Laboratory, said 'AMP2 promises a breakthrough to the problem of integrating spinal fusion implants with living tissues. Targeted delivery of biologics harnesses the excellent bone-forming capabilities of current biologics, while limiting off-target responses. Implants like OsteoAdapt SP simplify product preparation and, crucially, deliver an extremely high fusion rate as this study shows.'
About Theradaptive
Founded in 2016 and headquartered in Maryland, U.S., Theradaptive is a venture-backed biopharmaceutical and medical device company with the goal of leveraging its therapeutic delivery platform to deliver biologics where they are needed in the body with high precision and persistence to address unmet medical needs. Theradaptive is led by CEO Luis Alvarez, Ph.D., and its innovative platform has started to enable new therapeutics in spine, orthopedic and soft tissue repair as well as targeted immuno-oncology.
Media Contact:
Serena Lertora
serena.lertora@theradaptive.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/theradaptives-targetable-protein-amp2-beats-spinal-fusion-standard-of-care-301743550.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/theradaptives-targetable-protein-amp2-beats-spinal-fusion-standard-of-care-301743550.html
SOURCE Theradaptive