Thermedical®, a developer of thermal-ablation systems to treat ventricular arrhythmias, announced today that the U.S. Food & Drug Administration (FDA) has approved an open-label, single-arm interventional clinical trial to evaluate the safety and efficacy of the Thermedical® SERF Ablation System with the Durablate® Catheter in people with ventricular tachycardia (VT) resistant to conventional treatment.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20220822005129/en/
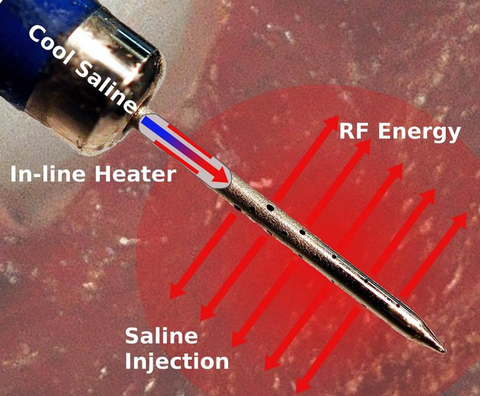
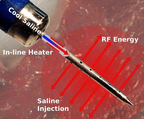
The U.S. FDA approved a clinical trial to evaluate the safety and efficacy of the Thermedical® SERF Ablation System with the Durablate® Catheter in people with ventricular tachycardia (VT) resistant to conventional treatment. VT is a leading cause of sudden cardiac death. (Photo: Business Wire)
SERF ablation with the Durablate catheter will be investigated as a treatment option for patients with ventricular arrhythmias resistant to antiarrhythmic drugs or standard ablation procedures. SERF ablation provides a new form of biological heat transfer designed to be more efficient than conventional ablation methods. The Durablate catheter has been developed to deliver energy with a high level of accuracy to better control the ablation size and treat tissue deeper in the heart wall where life-threatening arrhythmias that cause VT are often located.
“We are grateful to the FDA for the rapid approval of this pivotal trial to evaluate our Saline Enhanced Radiofrequency (SERF) Ablation System in patients who have run out of treatment options for their VT episodes and who suffer from extremely poor quality of life,” said Michael Curley, Ph.D., FHRS, co-founder and CEO of Thermedical, and senior author of the first-in-man multi-center trial evaluating SERF with the Durablate catheter, published in Circulation: Arrhythmia and Electrophysiology. “In our recent multi-center trial, 31 of 32 participants experienced immediate elimination of their clinical VT at the end of the procedure, and therapies such as shock or pace regulation were reduced by 89% during the five-month follow-up in these patients.”
The Mayo Clinic in Rochester, Minn., will be the first of up to 25 investigational sites in North America to enroll patients in the larger clinical trial. Douglas Packer, M.D., a cardiac electrophysiologist at Mayo Clinic, is the principal investigator. To date, additional sites are planned for Birmingham, Ala., Boston, Charleston, S.C., Montreal, Nashville, Tenn., Philadelphia, and Quebec City. The trial will enroll 154 participants with recurrent, sustained, monomorphic VT that is resistant to drug therapy and conventional catheter ablation. The trial participants will have a SERF ablation procedure, with follow-up at seven days, one month, three months, and six months. The Thermedical SERF Ablation System was designated as a Breakthrough technology in part because there is currently no approved device that specifically treats this patient population.
“This larger clinical trial is very important to further the evaluation of this innovative approach to treating problematic VT to reduce or eliminate shocks that implantable cardioverter defibrillators deliver to this patient population,” continued Dr. Curley. “We are encouraged by the early data and are eager to further demonstrate the safety and efficacy of our promising new SERF technology. Furthermore, SERF offers hope to people who suffer from this condition, as it could be life-changing for the treatment of refractory VT.”
Implantable Cardioverter Defibrillators (ICDs)—electronic devices that continually monitor a patient’s heart rhythm—are the current treatment for patients suffering from VT. During a VT episode, the ICD delivers energy to the heart muscle via a powerful shock or antitachycardia pacing to help the heart beat normally again. Today, VT patients with ICDs who experience VT episodes may be treated with conventional RF ablation, a lengthy procedure with a moderate success rate of approximately 50 percent.
About Thermedical
Thermedical is a privately held company founded by Massachusetts Institute of Technology (MIT) Hyperthermia Center alumni, Michael G. Curley, Ph.D. and Patrick S. Hamilton, Ph.D., based in Waltham, Mass. Under a Massachusetts Life Sciences Center Small Business Matching Grant (SBMG) Award, multiple NIH* Small Business Innovation Research (SBIR) Grants, and Series A venture funding, the company has developed thermal-ablation systems to treat ventricular tachycardia (VT) and solid tumors. For more information, visit www.thermedical.com.
*Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R44HL132746 and R44HL63535. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
View source version on businesswire.com: https://www.businesswire.com/news/home/20220822005129/en/
Source: Thermedical
Smart Multimedia Gallery
The U.S. FDA approved a clinical trial to evaluate the safety and efficacy of the Thermedical® SERF Ablation System with the Durablate® Catheter in people with ventricular tachycardia (VT) resistant to conventional treatment. VT is a leading cause of sudden cardiac death. (Photo: Business Wire)