Phase 3 confirmatory trials evaluate the safety and efficacy of using cryopreserved umbilical cord to achieve complete wound closure of non-healing DFUs with high-risk factors
Phase 3 confirmatory trials evaluate the safety and efficacy of using cryopreserved umbilical cord to achieve complete wound closure of non-healing DFUs with high-risk factors
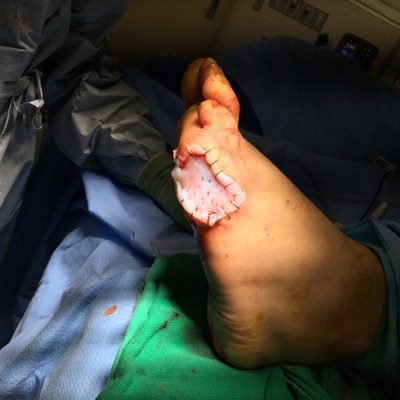
MIAMI, Nov. 12, 2020 /PRNewswire/ -- TissueTech, Inc., a pioneer in advanced wound care using cryopreserved amniotic membrane and umbilical cord birth tissue allografts, announced today the initiation of their second randomized controlled trial to study the benefits and risks of using therapeutic biologic product TTAX01 to achieve complete wound closure of advanced non-healing DFUs.
TissueTech is presently the only emerging biotechnology company in the U.S. to conduct two multi-center Phase 3 confirmatory random controlled trials at 40 investigational sites nationwide to research the treatment of advanced DFUs. These DFUs all present with high-risk ulcer depth factors indicating exposed bone, tendon, muscle, joint capsule, and clinical suspicion of osteomyelitis.
"TissueTech has pioneered the study of the natural healing elements of amniotic membrane and umbilical cord tissue-based products for more than 30 years. As such, we have been diligent in building an infrastructure to develop products as biologics under Section 351 for several years now," said TissueTech co-founder Amy Tseng, President, and Chief Executive Officer. "We determined early on that going after BLA approval in target indications such as wound care would be the right thing to do, as evidenced by the success of earlier clinical trials."
A previous one-year follow-up Phase 2 study using TTAX01 to treat difficult DFUs showed complete wound closure for 91% of patients with an average of only 1.5 applications. "A lower application rate required with TTAX01 is significant, especially during the COVID-19 pandemic where in-office visits are being reduced to lessen a patient's potential risk of virus exposure," said Herbert Slade, MD, , Chief Medical Officer at TissueTech.
TissueTech is also helping investigators prioritize their patients' safety with risk-based monitoring tools and centralized oversight. One example of this is TissueTech's recent adoption of an iPhone-based telehealth platform to accurately capture wound images and measurements in a validated and reproducible way. Clinical teams are optimizing a unified platform approach to capture clinical data and leveraging these technologies to support virtual clinical trial oversight.
"The COVID-19 pandemic has been a catalyst for change. Almost every aspect of clinical trials has been affected by CFR part 11 per U.S. Food and Drug Administration (FDA) guidance issued in March 2020 as a result of the pandemic," said Tommy Lee, Vice President of Clinical Operations for TissueTech. "We have been closely monitoring what is happening across the life science industry while communicating with the FDA and trial investigators, to understand these new guidelines and implement appropriate clinical contingency plans. As a result, we have adapted quickly to this new operating environment while ensuring the health and safety of our patients participating in these important investigational studies."
About TTAX01
TTAX01 is a code name for the cryopreserved human Amniotic Membrane and Umbilical Cord (AM/UC) matrix intended for use as a wound covering for dermal ulcers and defects. It is manufactured under Good Manufacturing Practice (GMP) regulations.
About TissueTech, Inc.
TissueTech, Inc., the parent company of Amniox Medical, Inc. and Bio-Tissue, Inc., is a scientific and market leader in the field of regenerative medicine. TissueTech manufactures a broad range of ocular, surgical, wound care, and soft tissue products that are marketed under these subsidiaries. Since the company's inception, clinicians have performed more than 500,000 human implants of the company's products and published more than 360 peer-reviewed studies supporting its platform technology. TissueTech is committed to an unwavering culture of integrity that places our patients' safety and clinical outcomes above all else. Learn more at https://tissuetech.com/.
View original content to download multimedia:http://www.prnewswire.com/news-releases/tissuetech-furthers-late-phase-research-of-biologic-candidate-ttax01-to-treat-advanced-wagner-grade-3-4-diabetic-foot-ulcers-301172052.html
SOURCE TissueTech