OrthoTrophix, Inc. announced that the Company has demonstrated evidence for disease modification in knee osteoarthritis in patients treated with TPX-100, the Company’s leading candidate for a Disease Modifying Osteoarthritis Drug.
OAKLAND, Calif., March 2, 2020 /PRNewswire/ -- OrthoTrophix, Inc., a privately held biopharmaceutical company, announced today that the Company has demonstrated evidence for disease modification in knee osteoarthritis (OA) in patients treated with TPX-100, the Company's leading candidate for a Disease Modifying Osteoarthritis Drug (DMOAD).
In a Phase 2, randomized, double-blind clinical trial, patients with mild to severe knee OA in both knees were treated with TPX-100 in one knee and identical placebo in the other knee. Drug and placebo were administered by intra-articular injection on days 0, 7, 14, and 21. MRI images of both knees were obtained at baseline, 6 months and 12 months. Clinical benefits including knee function and pain were measured by the KOOS (Knee Injury and Osteoarthritis Outcome Score) and WOMAC scales (Western Ontario and McMaster University Osteoarthritis Index). No additional treatment was given after the last (fourth) injection.
TPX-100-treated knees demonstrated clinically meaningful and statistically significant improvement in critical knee functions, based on 17 measures, as compared to placebo-treated knees by 6 months, with benefits maintained through 12 months (Figure 1).
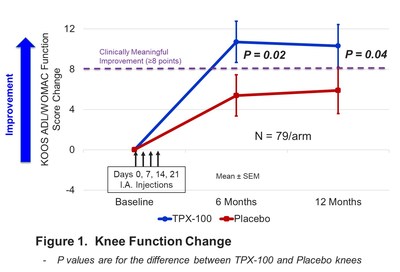
During the same period, pathological three-dimensional (3D) shape changes in the femur in TPX-100-treated knees were significantly reduced as compared to placebo-treated knees (Figure 2). When compared with data from the Osteoarthritis Initiative, a study of over 9,000 healthy and OA knees, the rate of femoral bone shape change of TPX-100-treated knees at 6 months was similar to that of healthy (non-OA) knees, while that of placebo-treated knees was consistent with progressive OA. Significant treatment differences in favor of TPX-100 were sustained through the end of the study at 12 months.
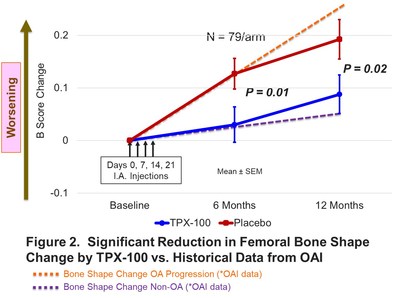
"This is a striking finding," commented Dr. Dawn McGuire, OrthoTrophix' Chief Medical Officer. "Femoral bone shape change has been shown to predict OA onset, progression and the need for knee replacement. The fact that TPX-100 treatment also was associated with marked and sustained clinical improvements suggests the relevance of normalizing bone shape change in mediating OA symptoms. With regard to pain and bone shape, as presented at the November, 2019 Annual Meeting of the American College of Rheumatology, TPX-100 treatment was associated with a substantial reduction in knee pain frequency, which correlated significantly with reduced patellar bone shape change. Collectively, the concordance between clinical and structural benefits is consistent with real disease modification by TPX-100. We are very enthusiastic about the potential of TPX-100 for the treatment both of disabling symptoms and underlying pathology in knee OA."
About OrthoTrophix, Inc.
OrthoTrophix, Inc., based in Oakland, California, is a privately held biopharmaceutical company focused on development and commercialization of a new class of Disease Modifying Osteoarthritis Drug (DMOAD). Founded by three co-founders in 2011, the primary focus of OrthoTrophix has been regeneration and repair of cartilage and underlying bones in the knee and other joints with its novel proprietary compounds.
This press release contains "forward-looking" statements. These statements involve risks and uncertainties, which may cause results to differ materially from those set forth in the statements. The forward-looking statements include statements regarding product development and cannot be guaranteed. OrthoTrophix undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Forward-looking statements in this press release should be evaluated together with the many uncertainties that affect OrthoTrophix' business.
Company Contact
Yoshi Kumagai
President and CEO
Tel: (510) 488-3824

![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/tpx-100-orthotrophix-demonstrates-evidence-for-disease-modification-in-knee-osteoarthritis-301013636.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/tpx-100-orthotrophix-demonstrates-evidence-for-disease-modification-in-knee-osteoarthritis-301013636.html
SOURCE OrthoTrophix, Inc.




