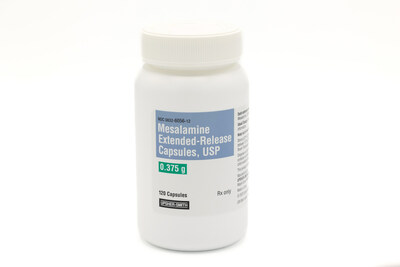
Upsher-Smith Laboratories, LLC (Upsher-Smith) today announced the recent launch of Mesalamine Extended-Release Capsules, USP 375 mg.
|
MAPLE GROVE, Minn., Aug. 21, 2023 /PRNewswire/ -- Upsher-Smith Laboratories, LLC (Upsher-Smith) today announced the recent launch of Mesalamine Extended-Release Capsules, USP 375 mg. The mesalamine capsule market had U.S. sales of approximately $105 million for the 12 months ending June 2023 according to IQVIA. Upsher-Smith's Mesalamine Extended-Release Capsules are AB-rated to APRISO® (mesalamine) extended-release capsules. Product Information
For questions about ordering, please call Upsher-Smith at 1-800-654-2299. Please refer to the full Prescribing Information for Mesalamine Extended-Release Capsules, USP here. You can also call 1-888-650-3789 to obtain a copy of the full Prescribing Information. You are encouraged to report suspected adverse reactions to Upsher-Smith Laboratories, LLC at 1-855-899-9180 or to the FDA by visiting http://www.fda.gov/medwatch. About Upsher-Smith *APRISO is a registered trademark of Salix Pharmaceuticals, Ltd.
SOURCE Upsher-Smith Laboratories, LLC |
||||||||||||||