Utah
Looking for a research and development job? Check out the BioSpace list of 12 companies hiring life sciences professionals like you.
In 2025, made or projected biopharma workforce cuts affected about 42,700 employees, according to BioSpace tallies. BioSpace takes a deep dive into which companies and locations were impacted and speaks to experts about what to expect ahead—and why.
Looking for a job in oncology? Check out the BioSpace list of nine companies hiring life sciences professionals like you.
The number of employees laid off and companies letting people go increased year over year during the first half of 2025. BioSpace recaps the five largest layoff rounds, including cuts at Bayer, BMS and Teva.
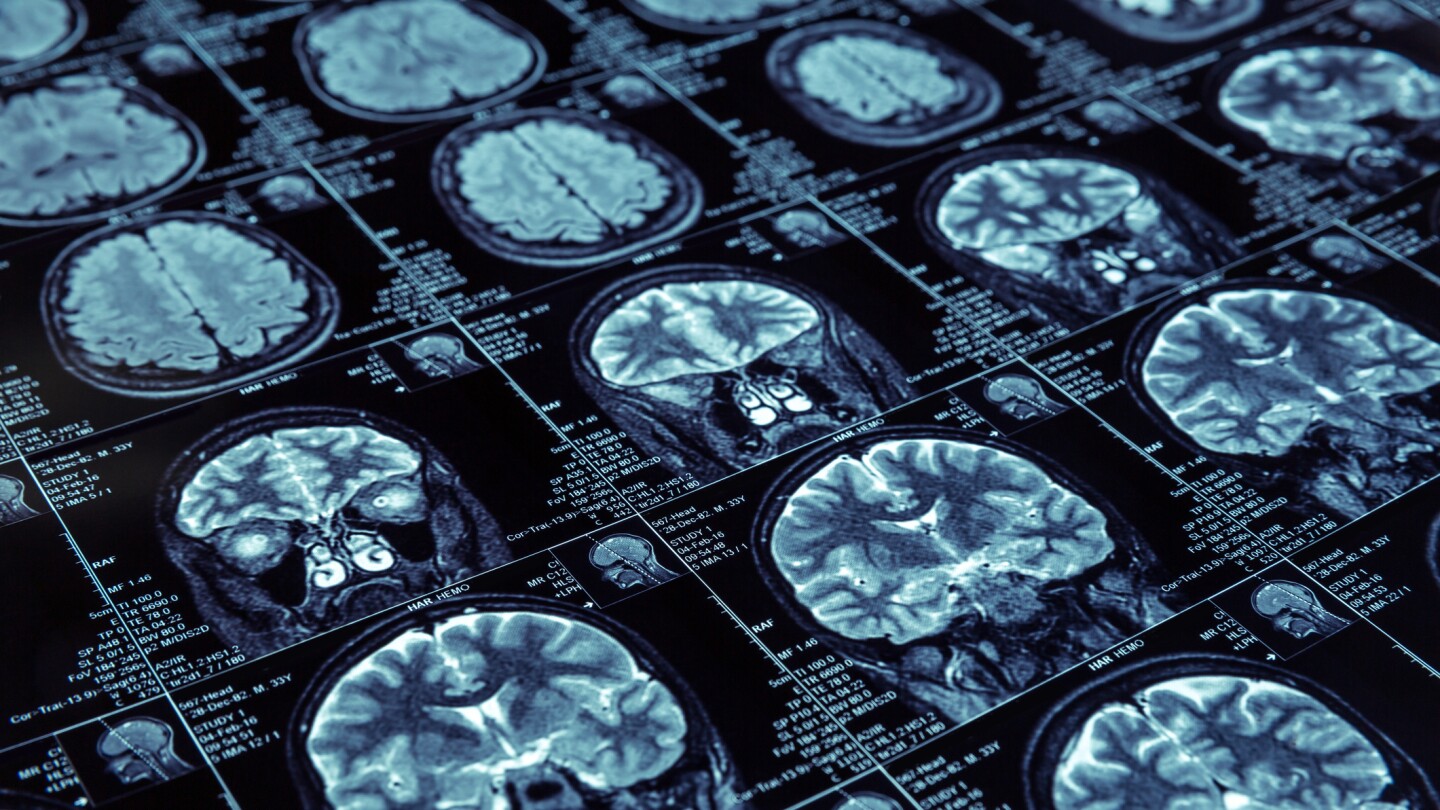
Recursion’s oral drug candidate for cerebral cavernous malformation showed no improvements in patient- or physician-reported outcomes at 12 months. The biotech will engage with the FDA to determine the need for an additional study.
Armed with a combined $850 million in cash, the companies said Thursday the resulting biotech will have a pipeline that could deliver 10 clinical readouts over the next 18 months.
PRESS RELEASES