Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today provided an update on its development program for tradipitant
|
WASHINGTON, Aug. 31, 2020 /PRNewswire/ -- Vanda Pharmaceuticals, Inc. (Vanda) (Nasdaq: VNDA) today provided an update on its development program for tradipitant.
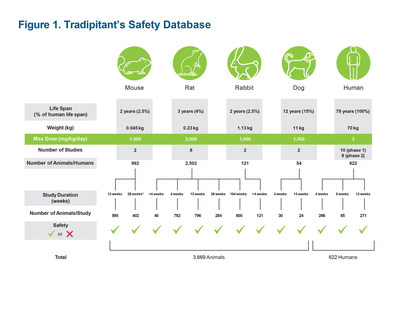
Clinical progress of tradipitant in gastroparesis Tradipitant is advancing in a Phase III study for the treatment of both diabetic and idiopathic gastroparesis. This study is 30% enrolled at a target of 200 randomized patients, and is expected to complete enrollment in the first half of 2021. The previous Phase II study showed significant improvement in nausea, the key symptom of gastroparesis, as well as significant improvement in the number of nausea-free days, in patients treated with 85 mg of tradipitant twice a day for 4 weeks. The Phase III study is a 12-week study of similar design to the Phase II study in both target population and endpoints. The article "Efficacy and Safety of Tradipitant in Patients with Diabetic and Idiopathic Gastroparesis in a Randomized, Placebo-Controlled Trial" was accepted for publication in Gastroenterology. A preprint is available online here. The article describes the results of Vanda's Phase II double-blind trial of 152 adults with gastroparesis at 47 sites in the U.S. from November 2016 through December 2018. Patients receiving tradipitant had a significant decrease in nausea score (reduction of 1.2) at week 4 compared with placebo (reduction of 0.7) (p=.0099), and a significant increase in of nausea-free days at week 4 (28.8% increase on tradipitant vs 15.0% on placebo; p=.0160). Patients with nausea and vomiting at baseline (n=101) had an even greater decrease in nausea when given tradipitant (reduction of 1.4) compared with those given placebo (reduction of 0.4) (p<.0001), as well as an increase in nausea-free days at week 4 (32.3% improvement on tradipitant vs 7.6% on placebo; p=.0003). The average nausea score was 1 or less at week 4 in 32.9% of patients given tradipitant compared with 11.8% of patients given placebo (p=.0013). A greater than 1-point improvement in the gastroparesis cardinal symptom index score was observed in 46.6% of patients given tradipitant compared with 23.5% of patients given placebo (p=.0053). In July 2020, the U.S. Food and Drug Administration (FDA) approved the use of tradipitant for up to 6 months with an option of renewal for an individual patient who requested expanded access. Since then, other patients who experienced a unique benefit in tradipitant studies have requested expanded access and their applications are currently under review by the FDA. Although this expanded access program is not intended primarily for data collection, Vanda will collect safety data from this cohort of expanded access patients and include this data in its New Drug Application (NDA) for gastroparesis. Preclinical and safety data for tradipitant A robust package of preclinical work for tradipitant has been completed, including a 2-year carcinogenicity study, depicted in Figure 1. Figure 1 also includes a summary of the human studies through Phase II. The results from these animal and human studies demonstrate tradipitant's extensive animal and human testing, and well-established safety profile. No significant safety signals were observed in these studies that would be both predictive of clinical safety and would preclude all further clinical development. In the human studies, tradipitant was well tolerated in individuals who received daily doses of tradipitant ranging from less than 50 mg/day to over 170 mg/day. Vanda believes this entire preclinical package is adequate to support NDA filings for the short-term indications being pursued. The FDA has communicated, however, that for treating patients beyond 12 weeks, it is requiring Vanda to conduct what it considers to be a standard 9-month non-rodent chronic toxicity study, which currently limits Vanda's ability to collect safety data in humans for more than 12 weeks. The FDA-required study design necessitates the sacrifice of dozens of animals and Vanda has disputed the necessity of a 9-month non-rodent chronic toxicity study. Vanda has taken a public position that unnecessary lethal animal studies should not be conducted, especially in dogs, as such studies are not scientifically justified and would not be consistent with Vanda's scientific and ethical principles, which are shared by many Americans. Vanda is working with the FDA to resolve this disagreement. Despite the disagreement with the FDA, the preclinical package has allowed Vanda to continue to conduct all the efficacy studies necessary for NDA filing. The lack of long-term (>12 weeks in humans) safety data would likely impact the FDA's willingness to approve tradipitant for a chronic indication. However, because long-term safety data is not normally a requirement for short-term indications, and with a preclinical profile that has not precluded clinical development, Vanda believes the package is complete for any NDA filing to treat patients for 12 weeks or less. In gastroparesis, for example, the FDA has communicated to Vanda that it is considering an indication for the short-term relief of nausea in gastroparesis. While this short-term indication is not preferred, Vanda would consider accepting this limited indication while continuing to pursue a chronic indication. The chronic treatment of itch in atopic dermatitis would be expected to have a similar issue in review as gastroparesis. The indications of motion sickness and the acute respiratory distress (pneumonia) associated with COVID-19 are both short-term indications covering either short-term travel, in the case of motion sickness, or a week to several weeks course of treatment in the case of COVID-19 pneumonia. At this time, the COVID-19 pneumonia program is recruiting patients in the Phase III ODYSSEY study. Preparations for the motion sickness study have resumed and the study will commence when local restrictions related to the global COVID-19 pandemic are lifted. Recruiting in the atopic dermatitis study remains on hold. Potential market opportunity for gastroparesis Gastroparesis is a severe, significantly underdiagnosed disease with increased morbidity and mortality, representing a significant unmet medical need. The only FDA approved treatment currently available is metoclopramide, which carries a label restriction of up to 3 months treatment because of increased risk of Parkinson's-like symptoms that can be permanent. Off label treatments include erythromycin, botulinum toxin injections and gastric stimulators, none of which provide a significant benefit. Anti-nausea agents, including ondansetron, are also used as needed, although with incomplete relief. As a result of this lack of effective therapeutic options, patients with gastroparesis experience a significant adverse impact on their social and occupational functioning in addition to physical symptoms. Given the magnitude of improvements seen in the Phase II study, tradipitant has the potential to become the first line option for the treatment of gastroparesis. As a result of underdiagnosis, it is difficult to estimate with any precision the number of patients that could be treated with tradipitant. IQVIA reports more than 300,000 monthly metoclopramide prescriptions, the equivalent of 300,000 patients treated with this drug, which is primarily used in the treatment of gastroparesis. However, the concentrated base of gastroenterology specialists and the development of new and easy to use diagnostics, such as a recently approved breath test that can be performed at home, along with Vanda's significant experience with patient-based marketing and direct-to-consumer awareness campaigns, could represent a large commercial opportunity for Vanda. Potential timeline and estimated milestones for tradipitant in gastroparesis
About Tradipitant Tradipitant is an NK-1R antagonist licensed by Vanda from Eli Lilly and Company. Tradipitant is currently in clinical development for gastroparesis, COVID-19 pneumonia, motion sickness and atopic dermatitis. The FDA has imposed a partial clinical hold on tradipitant clinical protocols of longer than 12 weeks duration. References 1 Carlin, J. L., Lieberman, V. R., Dahal, A., Keefe, M. S., Xiao, C., Birznieks, G., Abell, T. L., Lembo, A., Parkman, H., & Polymeropoulos, M. H. (2020). Efficacy and safety of tradipitant in patients with diabetic and idiopathic gastroparesis in a randomized, placebo-controlled trial. Gastroenterology. Advance online publication. https://doi.org/10.1053/j.gastro.2020.07.029 2 Refer to Company press release titled "Vanda Pharmaceuticals' Interim Analysis from ODYSSEY Study Shows Tradipitant may Accelerate Clinical Improvement in Patients with COVID-19 Pneumonia" issued on August 18, 2020. https://vandapharmaceuticalsinc.gcs-web.com/node/14256/pdf About Vanda Pharmaceuticals Inc. Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit www.vandapharma.com and follow us on Twitter @vandapharma. CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS Various statements in this press release, including, but not limited to statements regarding the completion of Vanda's Phase III study of tradipitant for the treatment of gastroparesis, the potential commercial opportunity for tradipitant, the ability of tradipitant to be a first line treatment option for patients with gastroparesis, the adequacy of Vanda's safety data and Vanda's pursuit of both long-term and short-term indications for gastroparesis, are "forward-looking statements" under the securities laws. Forward-looking statements are based upon current expectations that involve risks, changes in circumstances, assumptions and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda's forward-looking statements include, among others, Vanda's ability to complete enrollment for its Phase III study of tradipitant for the treatment of gastroparesis, Vanda's ability to complete the clinical development of, submit NDAs for and obtain regulatory approval of tradipitant in the treatment of gastroparesis, motion sickness and atopic dermatitis, Vanda's ability to resolve its disagreement with the FDA regarding the conduct of a 9-month non-rodent chronic toxicity study, and the FDA's assessment of the adequacy of Vanda's safety and efficacy data. There can be no assurance that the actual results or developments anticipated by Vanda will be realized or, even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Therefore, no assurance can be given that the outcomes stated in such forward-looking statements and estimates will be achieved. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda's business and market, particularly those identified in the "Cautionary Note Regarding Forward-Looking Statements", "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" sections of Vanda's Annual Report on Form 10-K for the fiscal year ended December 31, 2019, as updated by Vanda's subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov. All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. Corporate Contact: AJ Jones II Elizabeth Van Every
SOURCE Vanda Pharmaceuticals Inc. |
||
Company Codes: NASDAQ-NMS:VNDA |