Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration has cleared its Investigational New Drug (IND) application for VX-522, a messenger ribonucleic acid (mRNA) therapy targeted at treating the underlying cause of cystic fibrosis (CF) lung disease.
- Novel, inhaled mRNA therapy intended for the ~5,000 people with CF who cannot benefit from CFTR modulators -
- VX-522 clinical trial in people with CF to initiate in coming weeks -
BOSTON--(BUSINESS WIRE)-- Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) today announced that the U.S. Food and Drug Administration has cleared its Investigational New Drug (IND) application for VX-522, a messenger ribonucleic acid (mRNA) therapy targeted at treating the underlying cause of cystic fibrosis (CF) lung disease for the approximately 5,000 people with CF who cannot benefit from a cystic fibrosis transmembrane conductance regulator (CFTR) modulator. Vertex plans to initiate a single ascending dose clinical trial for VX-522 in people with CF in the coming weeks.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20221208005977/en/
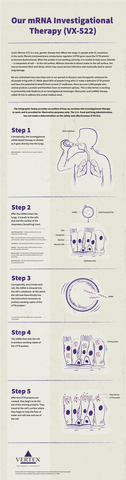
This infographic provides an outline of how we envision this investigational therapy to work and is provided for illustrative purposes only. (Graphic: Business Wire)
VX-522 is delivered to the lung through inhalation of a CFTR mRNA encapsulated by a lipid nanoparticle. Once delivered to the target lung cells, the mRNA is designed to produce functional copies of the CFTR protein. VX-522 is the result of an exclusive research collaboration established with Moderna in 2016.
“It has been our longstanding goal to bring highly effective therapies to all people with CF. Clearance of the IND represents a pivotal turning point in reaching the remaining ~5,000 people with CF who are still waiting for a medicine to treat the underlying cause of their disease,” said Reshma Kewalramani, M.D., FASN, Chief Executive Officer and President, Vertex. “The partnership with Moderna that began more than five years ago has been instrumental in achieving this milestone, and we look forward to continuing our work together.”
“This partnership brings together Vertex’s scientific expertise and decades of experience in developing cystic fibrosis medicines with Moderna’s proven leadership in mRNA technologies,” said Moderna Chief Executive Officer Stéphane Bancel. “Moderna’s development of a proprietary inhalable lipid nanoparticle to deliver a functional cystic fibrosis treatment to the lungs could lead to a transformational medical achievement. We are excited by the progress that has been made with the upcoming advancement of VX-522 to the clinic and look forward to our ongoing collaboration to develop treatments for the underlying cause of cystic fibrosis.”
About VX-522
This investigational messenger ribonucleic acid (mRNA) therapy aims to address the underlying cause of cystic fibrosis (CF). It is being evaluated by Vertex to treat lung disease for people living with CF who cannot benefit from cystic fibrosis transmembrane conductance regulator (CFTR) modulator treatments because they do not make any CFTR protein that responds to a CFTR modulator therapy. Globally, this represents a population of approximately 5,000 people living with this very rare form of the disease. The therapy is designed to deliver full length CFTR mRNA encapsulated in a lipid nanoparticle via inhalation directly to the lungs. Once delivered to target airway cells, the mRNA enables the production of functional CFTR protein. Improvements in CFTR quantity and function can lead to transformative benefits for people living with CF.
About the VX-522 Clinical Trial
Vertex plans to initiate a single dose escalation study in the coming weeks evaluating the safety and tolerability of VX-522 in people 18 years of age and older with cystic fibrosis and a CFTR genotype not responsive to CFTR modulator therapy.
About the Vertex and Moderna Collaboration
VX-522 is the result of an exclusive research collaboration Vertex established with Moderna in 2016 to discover and develop mRNA therapeutics for CF. Under the terms of the collaboration, Moderna leads asset identification efforts, combining its leading mRNA platform technology and mRNA delivery expertise together with Vertex’s scientific experience in CF biology, functional understanding of CFTR, and Vertex’s proprietary assay platform that utilizes human bronchial epithelial (HBE) cells of multiple different CF gene mutations from people with CF. Vertex leads preclinical and clinical development, regulatory and commercialization activities associated with the advancement of mRNA therapeutics that result from this collaboration and will fund all expenses related to the collaboration. Moderna is responsible for mRNA and lipid nanoparticle (LNP) process development and manufacturing. Per Vertex’s agreement with Moderna, Moderna will receive certain milestone payments, as well as royalty payments from this collaboration.
About Cystic Fibrosis
Cystic fibrosis (CF) is a rare, life-shortening genetic disease affecting more than 83,000 people globally. CF is a progressive, multi-organ disease that affects the lungs, liver, pancreas, GI tract, sinuses, sweat glands and reproductive tract. CF is caused by a defective and/or missing CFTR protein resulting from certain mutations in the CFTR gene. Children must inherit two defective CFTR genes — one from each parent — to have CF, and these mutations can be identified by a genetic test. While there are many different types of CFTR mutations that can cause the disease, the vast majority of people with CF have at least one F508del mutation. CFTR mutations lead to CF by causing CFTR protein to be defective or by leading to a shortage or absence of CFTR protein at the cell surface. The defective function and/or absence of CFTR protein results in poor flow of salt and water into and out of the cells in a number of organs. In the lungs, this leads to the buildup of abnormally thick, sticky mucus, chronic lung infections and progressive lung damage that eventually leads to death for many patients. The median age of death is in the early 30s.
About Vertex
Vertex is a global biotechnology company that invests in scientific innovation to create transformative medicines for people with serious diseases. The company has multiple approved medicines that treat the underlying cause of cystic fibrosis (CF) — a rare, life-threatening genetic disease — and has several ongoing clinical and research programs in CF. Beyond CF, Vertex has a robust clinical pipeline of investigational small molecule, cell and genetic therapies in other serious diseases where it has deep insight into causal human biology, including sickle cell disease, beta thalassemia, APOL1-mediated kidney disease, pain, type 1 diabetes and alpha-1 antitrypsin deficiency.
Founded in 1989 in Cambridge, Mass., Vertex’s global headquarters is now located in Boston’s Innovation District and its international headquarters is in London. Additionally, the company has research and development sites and commercial offices in North America, Europe, Australia and Latin America. Vertex is consistently recognized as one of the industry’s top places to work, including 13 consecutive years on Science magazine’s Top Employers list and one of Fortune’s Best Workplaces in Biotechnology and Pharmaceuticals and Best Workplaces for Women. For company updates and to learn more about Vertex’s history of innovation, visit www.vrtx.com or follow us on Facebook, Twitter, LinkedIn, YouTube and Instagram.
Special Note Regarding Forward-Looking Statements
This press release contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, statements made by Dr. Reshma Kewalramani and Stéphane Bancel in this press release, statements regarding our plans to initiate a clinical trial evaluating VX-522 in the coming weeks, the potential benefits of VX-522, including the related potential patient population, and our plans for the study design, including expectations for dosing. While Vertex believes the forward-looking statements contained in this press release are accurate, these forward-looking statements represent the company’s beliefs only as of the date of this press release and there are a number of risks and uncertainties that could cause actual events or results to differ materially from those expressed or implied by such forward-looking statements. Those risks and uncertainties include, among other things, that data from the company’s development programs, including programs with collaborators, may not support registration or further development of its compounds due to safety, efficacy or other reasons, that internal or external factors could delay, divert, or change our plans and objectives with respect to our research and development programs and/or our regulatory submissions, and other risks listed under the heading “Risk Factors” in Vertex’s most recent annual report filed with the Securities and Exchange Commission (SEC) and available through the company’s website at www.vrtx.com and on the SEC’s website at www.sec.gov. You should not place undue reliance on these statements or the scientific data presented. Vertex disclaims any obligation to update the information contained in this press release as new information becomes available.
(VRTX-GEN)
View source version on businesswire.com: https://www.businesswire.com/news/home/20221208005977/en/
Contacts
Vertex Pharmaceuticals Incorporated
Investors:
InvestorInfo@vrtx.com
or
Manisha Pai: +1 617-961-1899
Media:
mediainfo@vrtx.com
or
U.S.: +1 617-341-6992
or
Heather Nichols: +1 617-839-3607
or
International: +44 20 3204 5275
Source: Vertex Pharmaceuticals Incorporated







