DBV Technologies aims to fill the peanut allergy treatment gap, especially for highly allergic children, with their low dose epicutaneous immunotherapy (EPIT) patch Viaskin™ Peanut.
With only one FDA-approved therapy and standard-of-care treatment including avoidance and carrying an EpiPen in case of accidental exposure, the unmet need for peanut allergies is huge. Not to mention that peanut allergy is the most common food allergy in children, affecting 2.5% of U.S. children – nearly 1.8 million children and adults.
DBV Technologies aims to fill the peanut allergy treatment gap, especially for highly allergic children, with their low dose epicutaneous immunotherapy (EPIT) patch Viaskin™ Peanut.
“There’s an emotional aspect to the product - you’re putting this patch on your young child’s back every day instead of forcing them to eat something they might be dreading,” Pharis Mohideen, MD, Chief Medical Officer at DBV Technologies, told BioSpace. “It’s a nice feeling that you’re doing something you feel is going to make a difference and help them.”
Mohideen also has a unique perspective, wearing both clinical and personal hats. “I’m the father of two children who have food allergies, so I’ve been living this for about 15 or 16 years,” he added. “I think I have a slightly different perspective than the average chief medical officer.”
BioSpace spoke with Mohideen and Todd Green, MD, pediatric allergist and VP of Clinical Development and Medical Affairs at DBV, about their recently published Phase III open-label extension trial data, touching on the FDA’s recent decision to deny approval of Viaskin™ Peanut in its current form and what the next steps are. Rose Joachim, Ph.D., a Senior Healthcare Analyst at GlobalData, also provided insights into how Viaskin™ Peanut fits into the landscape of peanut immunotherapy.
Getting under the skin of peanut allergies
Remodeling an allergic immune response to a non-allergic one using immunotherapy is not a new concept – oral immunotherapy (OIT), where a prespecified amount of the allergen is consumed under medical supervision, has been used for years. But the first FDA-approved OIT, Aimmune Therapeutics’ Palforzia, was only just approved in January 2020.
Luckily, the peanut allergy drug development pipeline is bursting with creative immunotherapy options, including subcutaneous immunotherapy (allergen injected under the skin), epicutaneous immunotherapy (allergen administered selectively to the skin), and even a peanut protein-infused toothpaste for oral mucosal immunotherapy. EPIT is the furthest developed peanut immunotherapy in the pipeline, with DBV’s Viaskin™ Peanut patch leading the way.
“The simplicity and ease of use is really appealing to patients and their parents,” commented Mohideen. “In the COVID world we live in, it’s an added benefit that they would be able to stay out of the allergist’s office and do a telemedicine visit rather than have to come in to the office for scheduled updosing visits.”
The Viaskin™ Peanut patch consists of a quarter-sized foam ring with a plastic film on top. The peanut protein is on the inside of the plastic film, hovering above the skin but never directly touching.
Graphic of the Viaskin™ Peanut patch. Source: Dioszeghy V, et al. J Immunol. 2011;186:5629-5637. Mondoulet L, et al. Immunotherapy. 2015;7:1293-1305.
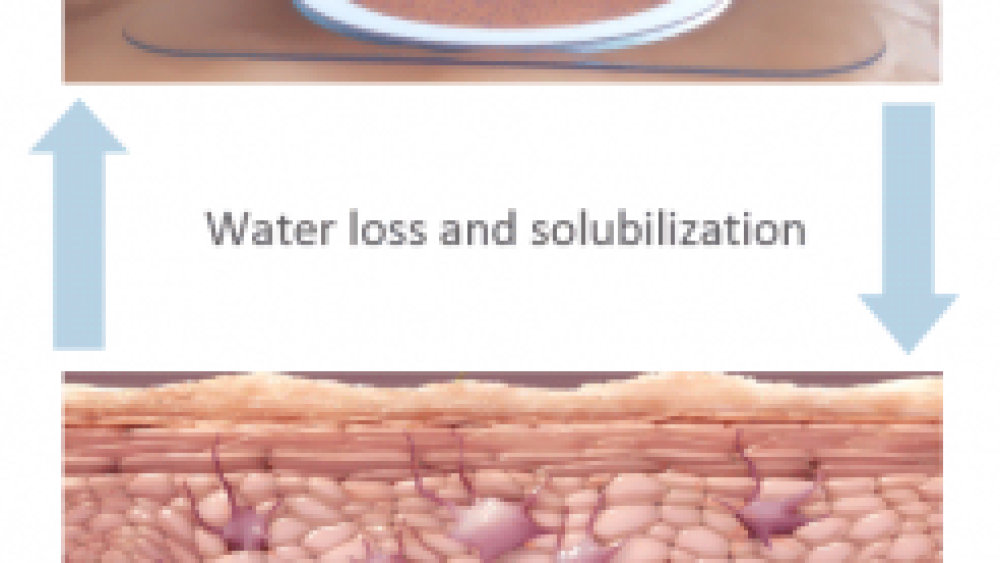
When the patch is worn, the bottom of the foam ring (which has adhesive on it) is stuck to the patient’s back, creating a condensation chamber. “Think back to the last time you had a Band-Aid on your finger and took it off the next morning – your skin was soft and moist, that’s precisely what happens here,” Mohideen explained. (And yes, the location of the patch affects its efficacy.)
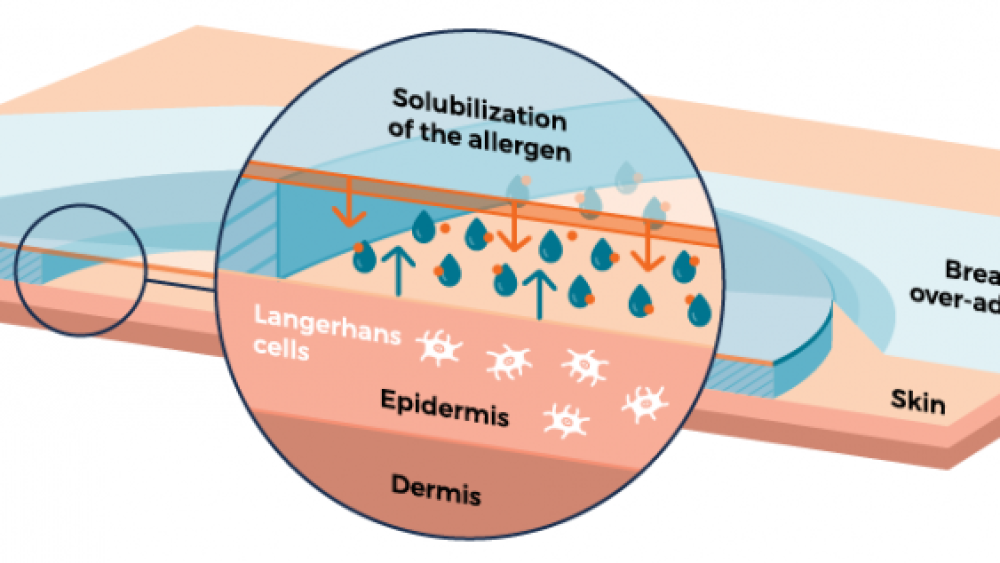
As the skin naturally loses moisture, water droplets form on the inside of the film, dissolving the peanut protein. The protein-containing droplets eventually fall off the film onto the skin and are absorbed.
The peanut allergen is solubilized from the patch and drops onto the skin. Source: DBV Technologies
“It is so ingenious, as far as the drug delivery system,” Mohideen commented. “It’s a really fascinating therapy quite unlike anything that is out there today.”
Once the peanut protein is on the skin, the immune system steps in, responding to the invading peanut protein. Specialized antigen presenting cells that patrol the skin, called Langerhans cells, begin the desensitization process. Although these cells travel throughout the body, there is little to no direct systemic absorption of the peanut protein. This is important for minimizing side effects and adverse reactions to the therapy.
The limited exposure of ultra-low doses of peanut protein make this a viable option for extremely sensitive allergy sufferers. “We’re talking about one 1,000th of a peanut kernel (250-300 micrograms), so just exquisitely minute quantities of peanut protein,” Mohideen added.
In fact, over the entire three-year follow-up of the PEOPLE study, patients who wore the Viaskin™ patch were only exposed to one peanut’s worth of peanut protein. That is a drastically lower exposure than what is generally considered low dose oral immunotherapy, which is about one peanut per day. For example, Palforzia’s daily dosing goes from 3 mg initially to a maintenance dose of 300 mg (about one peanut).
“It shows how potent the skin is as an immunomodulatory organ,” Green added.
Viaskin™ Peanut three years later
The PEOPLE Phase III trial is the open-label extension of the PEPITES Phase III trial. Their recently reported results involve the active arm from PEPITES, providing three years of treatment follow-up.
In the PEPITES trial, 356 children ages 4-11 years received either Viaskin™ Peanut or a placebo patch for one year. In the PEOPLE study, 298 PEPITES participants either elected to continue Viaskin™ Peanut treatment for an additional two years or receive Viaskin™ Peanut treatment for three years (if they were originally given the placebo patch).
Like other food allergy trials, PEPITES participants went through a double-blind, placebo-controlled food challenge (DBPCFC) to assess their sensitivity at the beginning and at various points during treatment to see how they were responding.
In a food challenge, each participant consumes certain amounts of peanut protein in a controlled setting with providers looking for predefined signs and symptoms of allergic reaction. The doses start small, gradually increasing with each dose – one milligram, three milligrams, etc. Once the participant shows the signs and symptoms of an allergic reaction, the food challenge is stopped. The highest dose they consumed is called the eliciting dose (the amount of peanut protein it took to provoke their symptoms). The total amount of peanut protein they consumed over the challenge is their cumulative reactive dose. The lower the dose, the more sensitive the patient is. So, increasing a person’s eliciting dose through treatment means they are less sensitive to peanuts – a key goal in reducing the risk of reacting to accidental peanut exposure.
The PEPITES study originally enrolled very sensitive participants, selecting children who reacted to a single peanut kernel or less (an eliciting dose of 300 mg or less).
The main take home conclusions from PEOPLE are encouraging. After three years of Viaskin™ Peanut treatment:
- 3 in 4 participants (76 percent) improved their peanut sensitivity (increased their eliciting dose) from baseline
- More than half (52 percent) of participants had a final eliciting dose of at least 1000 mg (about 3-4 peanuts) compared to 40 percent after 1 year of treatment
- On average, participants could tolerate almost 6 peanuts total (average 1,769 mg cumulative reactive dose) compared to less than a peanut (average 224 mg) before treatment
- About 1 in 7 participants (14 percent) could complete the food challenge after treatment, meaning they could eat almost an entire serving of peanut butter without having an allergic reaction (5444 mg of peanut protein, about 18 peanuts)
- There was a very high compliance rate (97 percent), extremely low drop-out rate due to adverse events (1 percent), and no treatment-related epinephrine used
“The PEOPLE trial drives home the fact that Viaskin™ Peanut is a very different approach to addressing peanut allergy when compared to oral immunotherapy; a difference that is an important strength for the product,” Joachim commented. “Although its overall efficacy, even after three years of therapy, is less dramatic than that seen with Aimmune’s oral immunotherapy agent, Palforzia, Viaskin™ Peanut has demonstrated significant utility in a substantial proportion of patients. Most importantly, it offers a unique dosing protocol and a solid safety profile, with the majority of patch-related side effects being limited to low-grade application site reactions. This could mean a more palatable first-line option for parents who are too anxious to start oral immunotherapy in their children or a second-line option for children who have tried oral immunotherapy and could not tolerate the side effects. Key opinion leaders interviewed by GlobalData stressed the diversity of their peanut allergy patients’ needs and advocated for the availability of as many safe and effective options as possible.”
Other positive results
The researchers also analyzed data from PEOPLE after they were collected (called post hoc analysis) to learn more and answer additional questions.
What predicted how well a participant responded to the patch (aka why did some people have higher eliciting doses at the end than others)?
- Patients who could tolerate the most peanut protein before treatment (had a higher eliciting dose) could tolerate more peanuts at the end as well (not surprisingly).
- Patients with lower peanut immunoglobulin E (IgE) levels at the beginning could eat more peanut protein at the end (had a higher eliciting dose) – generally, lower IgE levels mean less likelihood of reaction, meaning the children would be able to tolerate higher levels of peanut before reacting. “Peanut-specific IgE level can predict how likely you are to have some sort of reaction, although anybody, regardless of peanut IgE level, can have a bad reaction,” Green added.
- Counterintuitively, patients with more severe eczema had higher eliciting doses at the end of the study. Although the reason for this is unclear, Green and his colleagues speculate that it may be related to severe eczema patients having more efficient absorption of peanut protein through their skin.
- Younger patients (closer to 4 years old) had a more robust response compared to older patients (closer to 11 years old). Green points out that this is consistent with other peanut allergy studies that show allergic responses may be more modifiable earlier in life.
How did the length of daily patch wear affect the patch’s efficacy?
- There was no association between patches that detached before 24 hours and Viaskin™’s efficacy for 80 percent of participants.
- Children in the PEPITES trial wore the Viaskin™ patch for a median of 21.1 hours per day – 95 percent of Viaskin™ wearers wore their patch for more than 10 hours each day. The response rates increased slightly after more than 10 hours of wear (36.6 percent for >10 hours compared to 42.6 percent for >20 hours).
- Interestingly, participants with the highest patch detachment rates (prior to 24 hours) also tended to have higher initial peanut IgE levels and lower baseline eliciting doses. Even these most sensitive, hard-to-manage patients saw improved peanut tolerance. “You could try your most challenging, highest risk of reacting patients on the patch and have some reasonable level of confidence that they would be able to do well with that relative to some other therapies where even the starting dose may be a bit scary,” said Mohideen.
- In another small study, 30 non-allergic participants wore the patch for various durations from 2 to 24 hours and the amount of peanut protein left on the patches were assessed. Peanut protein on the Viaskin™ patch decreased steadily from 2-12 hours after application becoming nondetectable after 12 hours.
“Parents were encouraged to pick a time in the late afternoon or evening each day to apply the patch to their child,” said Green. “If the child is running around or swimming the next afternoon and it falls off, they don’t need to worry about immediately replacing it – they can just wait until their usual time to reapply a new patch.”
Joachim commented, “These are really crucial studies for DBV to be doing. A major concern of allergists interviewed by GlobalData regarding the use of Viaskin™ Peanut was the feasibility of getting a young, often very active, child to keep the patch on for a whole day. The fact that a child would receive nearly the entire dose after just 12 hours of wear is very useful information.”
How long could a participant remain desensitized after stopping initial treatment?
- 18 PEOPLE participants participated in an optional sustained unresponsiveness test, where they did a food challenge two months after stopping treatment to see if they were still less sensitive to peanuts.
- 14 of those participants (78.8 percent) maintained an eliciting dose of at least 1000 mg peanut protein (about 3-4 peanuts).
Looking forward
The PEOPLE extension study is scheduled to continue for another two years, for a total of five years of Viaskin™ treatment. Two-month sustained unresponsiveness will be probed after four and five years of treatment.
“There are a ton of questions, like ‘Can you play with different scenarios of stopping treatment?’ or ‘Can you start introducing peanuts?’ Green posed. “Those are the kinds of questions that are really fun and exciting to think about that will be really important to answer in the future.”
Despite the added protection the Viaskin™ Peanut patch can provide, even if it does show long-term sustained unresponsiveness, it still should not be considered a cure.
“The important thing to realize is that the goal of this is not to go out and freely consume peanut butter or other peanut products, but to have some degree of protection by making people less sensitive,” said Green. “This treatment is akin to seat belts – just because you have a seatbelt doesn’t mean you start driving recklessly, disobey traffic signals, and feel invincible, but having that seatbelt gives you some peace of mind that if something happens outside your control, you’re less at risk. You still need to maintain avoidance and have your epinephrine with you, just in case.”
“Most families are not looking to go out and eat a peanut butter and jelly sandwich, they just want options and protection,” Mohideen commented. “This level of protection is absolutely life altering for a family. It opens up a whole different world of restaurants and travel, some of the smaller, simpler things in life that you may not realize that allergy families go through day in and day out.”
Viaskin™ Peanut is also being explored in younger children (ages 1-3 years) in an ongoing Phase III trial (EPITOPE) and an open-label extension trial (EPOPEX). “The theory is the younger you are, the more plastic your immune system is and, potentially, the more robust your immune response,” Mohideen added. “So, it will be interesting to see how that matches up with the 4-11-year-olds.”
“The large, landmark LEAP study that came out a few years ago showed that introducing peanuts early was better in terms of allergy prevention,” added Green. “Although our studies are in kids who are already allergic, I don’t think it would be surprising if we see even better responses in 1-3-year-olds, but it’s too early to say yet.”
A bump in the road to approval
Unfortunately, the FDA denied approval of Viaskin™ Peanut on August 4, issuing a Complete Response Letter to DBV. It cited issues with patch adhesion on efficacy and is calling for patch modifications before they will review the application again, meaning more clinical studies.
“We plan to request a meeting with the agency in the coming days to further understand the feedback and determine next steps. We will provide any updates as appropriate following meeting with FDA,” Daniel Tassé, Chief Executive Officer of DBV Technologies, told BioSpace. “We continue to believe in the importance of this potential treatment option for patients. Once we fully understand the FDA’s concerns and potential requirements, we will be in a better place to comment further.”
“DBV has a unique product that I think the company will continue to pursue,” Joachim added. “Based on available clinical data, Viaskin™ Peanut seems to offer incremental, albeit very real, protection to patients with life-threatening peanut allergies. And compared to other marketed and pipeline peanut allergen immunotherapy options, most being oral immunotherapy, it has a unique dosing method and side effect profile, which may make it a more palatable option for certain types of patients. There is lot of added complexity when developing a therapy that utilizes a completely novel method of administration. While these bugs will likely be worked out, it will inevitably push back approval and launch by a year or more.”