Levita Magnetics today announced that the first ever robotic-assisted surgical procedures have been performed using the company’s newest system in development, the Levita ® Robotic Program.
System is Designed to Deliver Triple Impact: Clinical Benefit to Patients, Better Visualization and Control to Surgeons, and Higher Efficiency to Hospitals
MENLO PARK, Calif.--(BUSINESS WIRE)--Levita Magnetics, a company with a mission to improve access to better surgery for more patients, today announced that the first ever robotic-assisted surgical procedures have been performed using the company’s newest system in development, the Levita® Robotic Platform. The first case was a reduced-incision laparoscopic cholecystectomy (gallbladder removal) completed by Dr. Ignacio Robles, a minimally invasive surgeon at Clínica INDISA in Santiago, as part of a current clinical study of the system in Chile.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20210706005211/en/
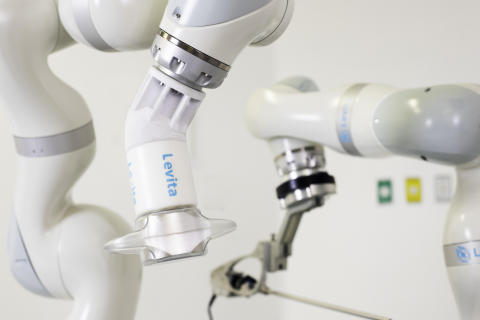
The Levita Robotic Platform is intended to deliver the clinical benefits of the company’s first commercial product, the Levita Magnetic Surgical System, including less pain, faster recovery and fewer scars for patients. (Photo: Business Wire)
The new robotic platform is intended to deliver the clinical benefits of the company’s first commercial product, the Levita® Magnetic Surgical System, including less pain, faster recovery and fewer scars for patients.1,2 The platform is intended to improve visualization, maintain surgeon control of instruments, and increase hospital efficiency with fewer assistive personnel required to conduct the procedures. With its compact footprint, the robotic platform is specially designed for high volume ambulatory or same-day discharge abdominal surgeries. The aim of these combined benefits is to increase the volume of high-quality, efficiently performed procedures while improving surgical access to patients.
“I am pleased to have the opportunity to perform the world’s first procedures using this innovative robotic platform. The first surgery was a challenging acute cholecystitis, nevertheless, the procedure went very smoothly and the patient had an outstanding recovery with no complications reported at 30 days post-procedure. Since then, we have performed two additional acute cholecystectomies using the robotic platform, with both patients enrolled and treated on the same day,” said Dr. Robles. “The combination of Levita’s magnetic technology within a robotic platform shows great promise to improve surgeon control and efficiency during surgical procedures. I’m excited to continue participating in this clinical study to further support the development of this platform.”
"Levita’s original Magnetic Surgical System has been reported to improve surgical outcomes for patients with less pain and fewer incisions,” said Steven D. Schwaitzberg, MD, FACS, Professor and Chair of the Department of Surgery at Jacobs School of Medicine and Biomedical Sciences, University at Buffalo. “Further advancing this technology into a robotic surgery platform has the potential to be useful in many procedures by providing a stable visualization platform without the need for an additional assistant.”
“We are taking Magnetic Surgery to the next level with this disruptive approach. Our robotic platform is designed to be the first to enable the clinical benefits of a less invasive procedure with fewer incisions, while allowing the surgeon full control of the platform and surgical instruments directly beside the patient,” said Dr. Alberto Rodriguez-Navarro, minimally invasive surgeon, founder and CEO of Levita Magnetics. “We believe enabling timely access to needed surgery has a direct impact on the quality of life for patients and may reduce the risk of complications or death due to the underlying disease. Our ultimate goal is to globally increase the ability of surgeons and hospitals to provide more patients access to better surgical procedures within their communities. We are advancing our clinical study and plan to submit the Levita Robotic Platform to the U.S. FDA for clearance in late 2021. We aim to deliver what society is expecting from surgical robotics.”
About the Levita® Robotic Platform
The Levita Robotic Platform is an investigational system. It is not available for commercial sale in the United States and has not been cleared by the Food and Drug Administration (FDA).
About the Levita® Magnetic Surgical System
The Levita Magnetic Surgical System enables reduced-port laparoscopic surgical procedures designed to minimize the footprint of surgery. The system, which consists of an external magnet placed on the skin that controls a shaftless detachable grasper, enables instruments to move without the constraints of a fixed-position pivot point. The Levita Magnetic Surgical System is indicated in the U.S. to grasp and retract the body and the fundus of the gallbladder in laparoscopic cholecystectomy procedures; the liver in bariatric procedures; the prostate and periprostatic tissue in prostatectomy procedures; and the colon, rectum, and peri-colorectal tissue in colorectal procedures to facilitate access and visualization of the surgical site. The device is indicated for use in patients with a body mass index (BMI) range of 20 to 60 kg/m2.
About Levita® Magnetics
Headquartered in Silicon Valley, Levita Magnetics developed the first and only Magnetic Surgery® platform, the Levita Magnetic Surgical System. Founded by innovator and surgeon Dr. Alberto Rodriguez-Navarro, this proprietary technology is designed to evolve minimally invasive surgery with the goal of minimizing the footprint of surgery and improving patient outcomes. It is the first magnetic surgical system to receive FDA clearance. For more information visit www.magneticsurgery.com and www.levita.com.
1. Welsh LK, et al. Magnetic Liver Retraction Decreases Postoperative Pain and Length of Stay in Bariatric Surgery Compared to Nathanson Device. J Laparoendosc Adv Surg Tech A. 2021 31(2):194-202
2. Rivas H, et al. Magnetic Surgery: Results From First Prospective Clinical Trial in 50 Patients. Annals of Surgery, 2018,267(1):88-93.
View source version on businesswire.com: https://www.businesswire.com/news/home/20210706005211/en/
Source: Levita Magnetics







